Brain Multi-Omic Subtypes of Neuroticism Reveal Molecular Signatures linked to Alzheimer's Disease
Brain Multi-Omic Subtypes of Neuroticism Reveal Molecular Signatures linked to Alzheimer's Disease
Zammit, A. R.; Lopes, K. d. P.; Batalha, C. M. P. F.; Yu, L.; Poole, V. N.; Tasaki, S.; Kapasi, A.; Wang, Y.; De Jager, P. L.; Menon, V.; Seyfried, N.; Kaddurah-Daouk, R.; Iturria-Medina, Y.; Bennett, D. A.
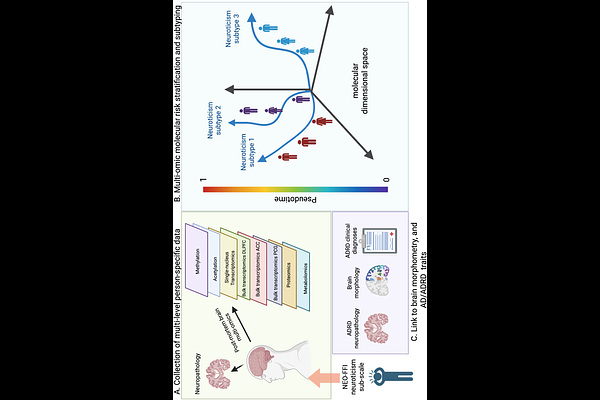
AbstractImportance: Molecular mechanisms linking neuroticism with Alzheimer\'s disease traits are unknown. Objective: To identify molecular subtypes of neuroticism and examine their association with ADRD traits. Design: Three ongoing cohort studies were used; Religious Orders Study (ROS), Rush Memory and Aging Project (MAP) and Minority Aging Research Study (MARS), that began enrollment in 1994, 1997, and 2004, respectively. Setting: Older priests, nuns, and brothers from across the U.S. (ROS), older adults (MAP) and older African-American adults (MARS) from across the greater Chicago metropolitan area. Participants: 1,028 decedents with multi-omic data from the dorsolateral prefrontal cortex (DLPFC), the anterior cingulate cortex (AC), and the posterior cingulate gyrus (PCG). Exposure(s): Eight layers of omics (DNA methylation and histone acetylation from DLPFC; RNA seq from AC, DLPFC, and PCG, single-nucleus RNA, TMT proteomics and metabolomics from DLPFC) and Neuroticism using the 12-item version from the NEO Five-Factor Inventory. Main outcome(s) and measure(s): Person-specific multi-omic molecular pseudotime representing molecular progression from low to high phenotypic expression of neuroticism, and three multi-omic brain molecular subtypes of neuroticism which represent distinct omic pathways from no/low neuroticism to high neuroticism that differ by their omic constituents. Participants are exclusively assigned to the subtype which aligns mostly with their multi-omic profile. Results: The top drivers of subtype differentiation were transcriptomic alterations across three brain regions (DLPFC, AC, PCG) which extensively and differentially characterized the subtypes. The subtypes were also differentially associated with AD pathology, temporal lobe atrophy, and AD dementia, with subtype N1 showing the strongest associations. Conclusions and Relevance: Neuroticism may be driven by three distinct molecular subtypes, with subtype N1 driving ADRD-related associations, N2 showing some ADRD associations, and N3 being completely independent of these outcomes. Our data provide novel insights into the biology of individual differences in predispositions of neuroticism and its associations with ADRD traits.