Structural insights into Cas9-mediated prespacer selection in CRISPR-Cas adaptation
Structural insights into Cas9-mediated prespacer selection in CRISPR-Cas adaptation
Gaizauskaite, U.; Tamulaitiene, G.; Silanskas, A.; Gasiunas, G.; Siksnys, V.; Sasnauskas, G.
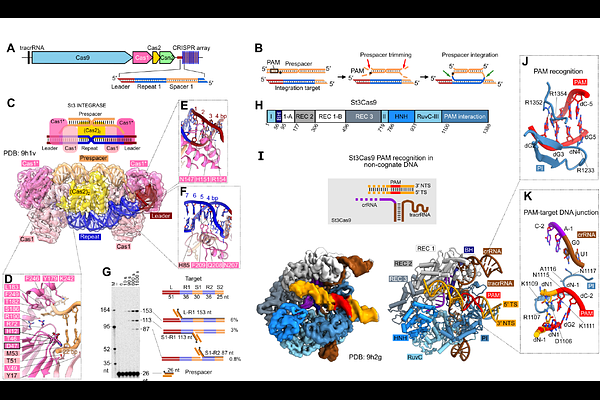
AbstractDuring CRISPR-Cas adaptation, prokaryotic cells become immunized by the insertion of foreign DNA fragments, termed spacers, into the host genome to serve as templates for RNA-guided immunity. Spacer acquisition relies on the Cas1-Cas2 integrase and accessory proteins like Cas4, which select DNA sequences flanked by the protospacer adjacent motif (PAM) and insert them into the CRISPR array. It has been shown that in type II-A systems selection of PAM-proximal prespacers is mediated by the effector nuclease Cas9, which forms a \'supercomplex\' with the Cas1-Cas2 integrase and the Csn2 protein. However, the supercomplex structure and the role of the ring-like Csn2 protein remain unknown. Here, we present cryo-electron microscopy structures of the type II-A prespacer selection supercomplex in the DNA-scanning and two different PAM-bound configurations. Our study uncovers the mechanism of Cas9-mediated prespacer selection in type II-A CRISPR-Cas systems, and reveals the role of the accessory protein Csn2, which serves as a platform for the assembly of Cas9 and Cas1-Cas2 integrase on prespacer DNA, reminiscent of the sliding clamp in DNA replication. Repurposing of Cas9 by the CRISPR adaptation machinery for prespacer selection characterized here demonstrates Cas9 plasticity and expands our knowledge of the Cas9 biology.