SHIP1 regulates TREM2 signalling and macrophage functions in a hiPSC-derived model
SHIP1 regulates TREM2 signalling and macrophage functions in a hiPSC-derived model
Martin, C. S.; Obst, J.; Ibarra, S.; Murphy, E.; Gospodinova, K. O.; Mead, E.
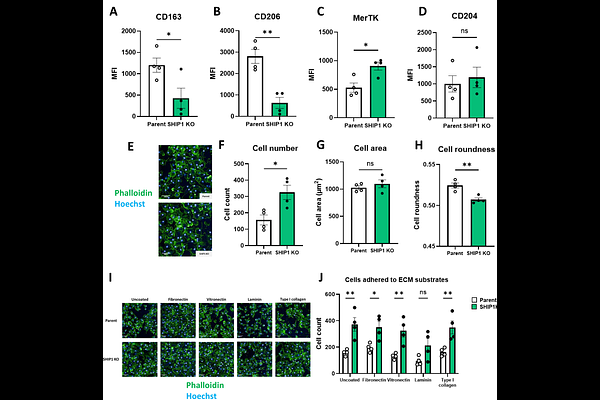
AbstractGenetic and functional studies strongly implicate microglia in the pathology of Alzheimers disease (AD). The Triggering Receptor Expressed on Myeloid cells 2 (TREM2) pathway is an important functional regulator of microglia in AD, promoting phagocytosis of apoptotic cells, debris and pathogenic proteins including amyloid beta. Additionally, genome-wide association studies have identified risk bearing polymorphisms in several members of the pathway including INPP5D, encoding the inositol phosphatase SHIP1. Recent studies utilising in vitro macrophage and microglia models and preclinical mouse models have identified a role for SHIP1 in both modulating TREM2 signalling and modulating neurotoxic microglial activation. In this study, we characterised the role of SHIP1 in regulating TREM2 signalling and global microglial functions using human induced pluripotent stem cell (hiPSC)-derived macrophages as a physiologically-relevant in vitro model of microglia. Isogenic parent and SHIP1 knockout (SHIP1 KO) lines were generated and macrophage phenotype and functions investigated. Knocking out SHIP1 was associated with a decreased expression of the lipopolysaccharide (LPS) co-receptor CD14, which translated to lower secretion of pro-inflammatory cytokines on stimulation with LPS. Additionally, SHIP1 KO hiPSC-derived macrophages showed decreased signalling upon TREM2 stimulation, which was further reflected in a reduced level of phagocytosis of apoptotic neurons compared to the parental line. The observed attenuated TREM2 signalling did not correspond to decreased TREM2 protein levels nor increased shedding of the receptor. Examination of macrophage scavenger receptor expression revealed reduced cell surface levels of CD163 and CD206 involved in resolution of inflammation but increased levels of the phagocytic receptor MerTK, while morphological analysis revealed a less amoeboid activated phenotype and increased adhesion. Taken together, our data indicates that SHIP1 plays a key role in regulating global macrophage functions in addition to modulating TREM2 signalling and inflammation.