Loss of B cell tolerance at the T2/T3a B cell transition is a convergent pathogenic mechanism in common variable immunodeficiency
Loss of B cell tolerance at the T2/T3a B cell transition is a convergent pathogenic mechanism in common variable immunodeficiency
Hillier, K.; Yuen, G.; Hui, A.; Kumar, S.; Roy, P. G.; McColgan, J.; Zaldana, K.; Premo, K.; Kaneko, N.; Ingram, N.; Barmettler, S.; Ghebremichael, M.; Walter, J. E.; Perugino, C. A.; Das, J.; Farmer, J. R.; Pillai, S.
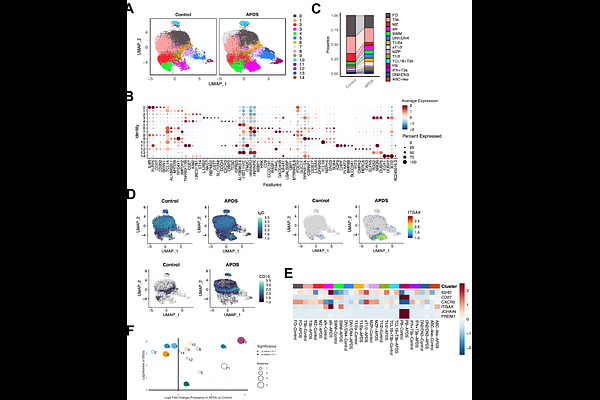
AbstractMany patients with common variable immunodeficiency (CVID), including those with CTLA4 deficiency, NFKB1 variants and activated PI3K-delta syndrome (APDS), develop autoimmunity that is refractory to treatment. Despite this shared clinical phenotype, a unifying mechanism for the breakdown of B cell tolerance across monogenic forms of CVID has not been established. Here, we demonstrate that patients with loss-of-function NFKB1 variants, like those with CTLA4 variants and APDS, exhibit dysregulated CD4+ T cell expansion, accumulation of transitional B cells, and a relative lack of follicular B cells. In patients with monogenic CVID and clinical autoimmunity, we observed a relative expansion of transitional and activated naive (aN: CD21loCD11chi) B cells in peripheral blood accompanied by a marked increase in the frequency of VH4-34 expressing autoreactive 9G4+ B cells, which expanded between T2 and T3a transitional B cell stages. Single-cell transcriptomic and B cell receptor analysis further revealed a marked expansion of activated T1/2, T3 and extrafollicular activated naive and double negative (DN: IgD-CD27-) B cell subsets in APDS patients. Notably, one B cell subset appeared exclusively in the APDS disease state, characterized by high oxidative phosphorylation in transitional B cells, specifically. In APDS patients, we also observed a clonal expansion of specific extrafollicular class-switched DN B cells, which were clonally derived from activated transitional B cells. DN B cells were also identified in APDS lung tissue, consistent with the contribution of activated, extrafollicularly-derived B cells to tissue inflammation. Together, these findings suggest that in many patients with CVID and autoimmune features, premature activation of autoreactive transitional T2 and T3a B cells induces the survival and expansion, rather than the tolerization and elimination, of self-reactive B cells. This process leads to extrafollicular expansion of autoreactive B cells capable of tissue infiltration.