Phase Separation Oppositely Modulates G-quadruplex and i-Motif DNA Folding in the Nuclei of Living Cells
Phase Separation Oppositely Modulates G-quadruplex and i-Motif DNA Folding in the Nuclei of Living Cells
Patel, B.; Hoang, C.; Yoo, H.; Davis, C.
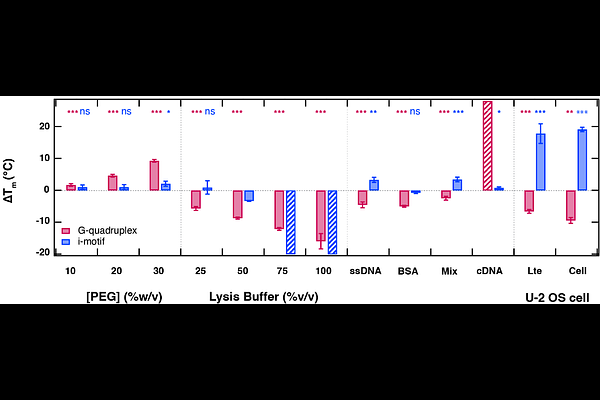
AbstractG-quadruplexes and i-motifs are non-canonical DNA structures that are thought to play an important role in the regulation of gene expression. Although these structures have been well studied in vitro, they are often characterized in non-physiological buffers intended to stabilize their structure. Therefore, additional work is necessary to understand how the cellular environment promotes their folding. Here we investigate the folding of the 15-mer thrombin- binding aptamer G-quadruplex (G4) and the designed 35-mer TAA(C5TAA)4 i-motif (iM) inside living osteosarcoma cells. We use fast relaxation imaging, which couples fluorescence microscopy with a laser-induced temperature jump, to quantify the stability and dynamics of FRET-labeled constructs. The melting temperature of G4 and iM inside cells are 44.9 {+/-} 0.9 {degrees}C and 46.5 {+/-} 0.6 {degrees}C, respectively. Compared to in vitro buffers, inside cells G4 is destabilized by {approx}10 {degrees}C and iM is stabilized by {approx}20 {degrees}C. These in-cell stabilities cannot be replicated by simple buffers that account for macromolecular crowding or non-specific interactions in the nuclear environment. Instead, specific interactions with nuclear proteins produce the observed in-cell stability trends. Furthermore, our results reveal that charge-driven condensation regulates the in-cell folding kinetics of both G4 and iM. Our work suggests that the kinetics of i-motif and G-quadruplex DNA are carefully tuned by phase separation to fit the seconds-to-minutes timescales of key regulatory processes inside cells. Taken together, our findings underscore the importance of studying the folding of DNA structures under near-physiological conditions to their mechanistic understanding as well as for the development of DNA-targeted drugs.