A biochemical mechanism for Stu2/XMAP215-family microtubule polymerases
A biochemical mechanism for Stu2/XMAP215-family microtubule polymerases
Gangadharan, B.; Kober, D. L.; Rice, L. M.
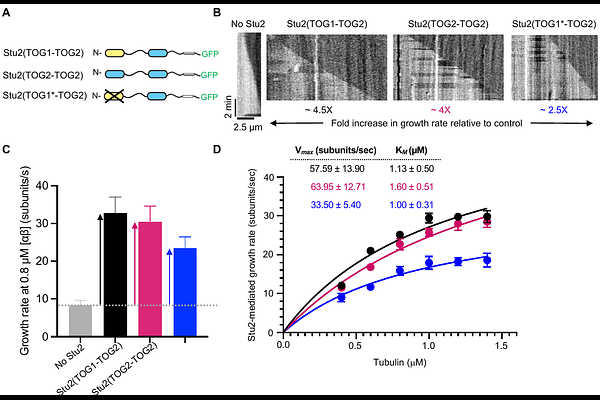
AbstractDefining quantitative biochemical mechanisms of microtubule dynamics and regulation is a current challenge. Stu2/XMAP215-family polymerases use tubulin-binding TOG domains to catalyze microtubule growth, but how polymerase activity results from the number and tubulin-binding properties of TOGs is not understood. We tested whether an enzyme-like biochemical model for the unrelated actin polymerase Ena/VASP could be applied to quantitatively relate Stu2 microtubule polymerase activity to the number of its TOGs and the rate constants governing their interactions with tubulin. Stu2 activity displayed enzyme-like characteristics consistent with the biochemical model: Stu2 stimulated microtubule growth rates with hyperbolic dependence on tubulin concentration, and the amount of Stu2 on the microtubule end did not vary with tubulin concentration (microtubule growth rate). Complementary measurements of TOG:tubulin binding revealed high affinity (10 nM) and slow dissociation (0.03 s-1). The polymerase and binding measurements can be unified within the biochemical model: Stu2 operates with high efficiency, acting as a tubulin-shuttling antenna on the microtubule end that is primarily limited by the rate of tubulin:TOG association. Our work thus provides a quantitative biochemical mechanism for TOG-based polymerases. That unrelated microtubule and actin polymerases use the same enzyme-like mechanism provides an example of convergent evolution in the cytoskeleton.