Nested mobile genetic elements mediating antimicrobial resistance genes mobility within and between cells in clinical and environmental Acinetobacter isolates
Nested mobile genetic elements mediating antimicrobial resistance genes mobility within and between cells in clinical and environmental Acinetobacter isolates
Bongulto, K.; Kagia, N.; Tauchi, H.; Suzuki, S.; Watanabe, K.
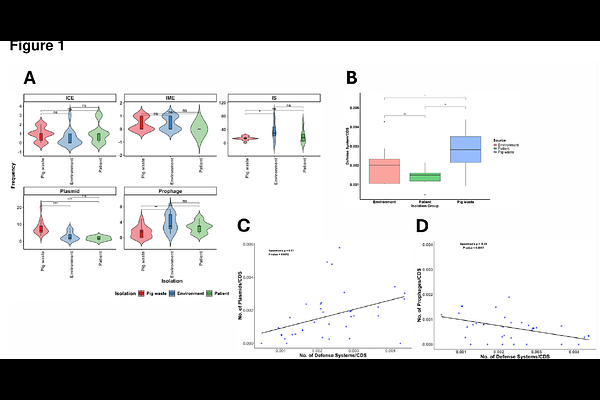
AbstractMobile genetic elements (MGEs) play a central role in the acquisition and dissemination of antibiotic resistance genes (ARGs). This study analyzed the distribution of MGEs using the whole-genome sequences of 38 Acinetobacter isolates from patient, environmental, and pig waste samples. Insertion sequence (IS) elements were identified as the most common MGE type, followed by plasmids and prophages, with significant variations depending on isolation sources. Pig waste isolates exhibited the highest mean number of plasmids, while prophages were more prevalent in environment-associated isolates. Interestingly, we observed a significant positive correlation between the number of plasmids and number of defense systems. In contrast, a significant negative correlation was identified between the number of prophages and number of defense systems. Plasmids were the primary vectors of ARGs. Co-localization of multiple ARGs, such as msr(E) and mph(E); blaOXA-58, tetY, mphB, and sul2 within a single plasmid was observed, suggesting potential for co-resistance and multidrug resistance. ARGs were enriched in pdif modules, with up to 10 distinct ARGs observed within a single module. Moreover, IS elements were more densely concentrated in conjugative elements. The study further highlights the role of nested MGEs in enabling hierarchical ARG transfer processes both within and between bacterial cells. Additionally, putative novel genomic resistance islands (GRIs) were identified in non-baumannii Acinetobacter species, representing the first documentation of GRIs outside the Acinetobacter calcoaceticus-baumannii (Acb) complex. This study provides new insights into the mechanisms of ARG dissemination in Acinetobacter, particularly the role of MGEs in facilitating hierarchical gene transfer processes.