Cancer-Associated USP28 Missense Mutations Disrupt 53BP1 Interaction and p53 Stabilization
Cancer-Associated USP28 Missense Mutations Disrupt 53BP1 Interaction and p53 Stabilization
Belal, H.; Ng, E. F. Y.; Ohta, M.; Meitinger, F.
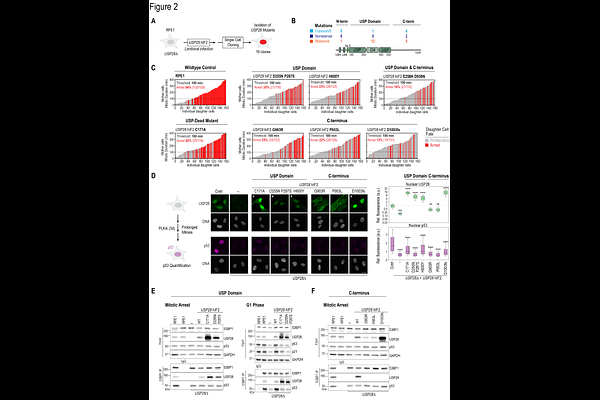
AbstractCellular stress response pathways are essential for genome stability and are frequently dysregulated in cancer. Following mitotic stress, the ubiquitin-specific protease 28 (USP28) and the p53-binding protein 1 (53BP1) form a stable, heritable complex to stabilize the tumor suppressor p53, triggering cell cycle arrest or apoptosis. Here we determine the mechanism by which USP28 stabilizes p53 and show that USP28 is required not only for an efficient stress response but also for maintaining basal p53 levels in some cancer cells. Loss of functional USP28 allows cells to evade mitotic stress and DNA damage responses in a manner that is specific to cell type and cancer context. We identify a prevalent, shorter USP28 isoform critical for p53 stabilization. Its C-terminal domain mediates PLK1-dependent binding to 53BP1, a dimerization-driven interaction necessary for mitotic stress memory, p53 stabilization, and cell cycle arrest. Cancer-associated missense mutations in this domain disrupt 53BP1 binding, impair nuclear localization, and destabilize USP28, compromising p53 stabilization. Notably, mutations in the 53BP1-binding domain occur more frequently in tumors than those in the catalytic domain, suggesting a potential role in cancer progression and implications for therapeutic strategies.