Entry Steps in the Biosynthetic Pathway to DiterpenoidAlkaloids in Delphinium grandiflorum and Aconitum plicatum
Entry Steps in the Biosynthetic Pathway to DiterpenoidAlkaloids in Delphinium grandiflorum and Aconitum plicatum
Miller, G. P.; Mutabdzija-Nedelcheva, L.; Andersen, T.; Pascoe, I.; Van Winkle, K.; Pluskal, T.; Hamberger, B.
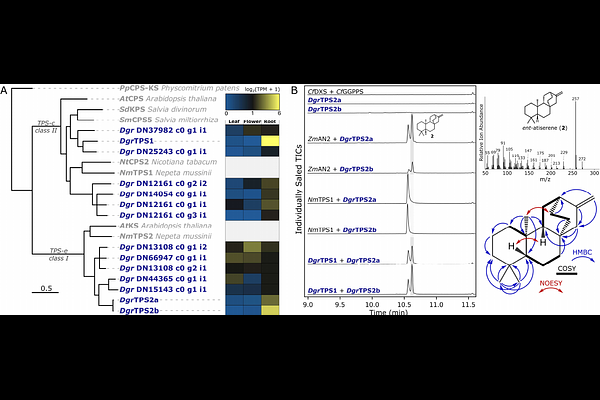
AbstractRoots from the Aconitum (Wolf\'s-Bane) and Delphinium (Larkspur) genera have been widely used in traditional medicine owing to the abundance of bioactive diterpenoid alkaloids that they produce. Despite extensive research on these compounds and their potential medicinal applications, their structural complexity precludes their production through total chemical synthesis, and little progress has been made towards elucidation of their biosynthetic pathways. Here, we report the entry steps in the biosynthesis of the diterpenoid alkaloid atisinium, constituting six enzymes identified from the Siberian Larkspur (Delphinium grandiflorum) and garden monkshood (Aconitum plicatum) through a combination of comparative transcriptomics between tissue types and genera and coexpression analysis. This pathway includes a pair of terpene synthases, three cytochromes P450, and a reductase with little homology to other characterized enzymes. We further demonstrate, through incorporation of isotopically labelled substrates, the preference of the reductase for ethanolamine over ethylamine, and similarly that ethanolamine is the preferred source of nitrogen for the majority of detected diterpenoid alkaloids. Identification of these enzymes and production of a key intermediate in a heterologous host paves the way for biosynthetic production of this group of metabolites with promise for medicinal applications.