A new FKBP51-GR-p23-Hsp902 multi-cochaperone complex identified and characterized by site-specific in-cell photocrosslinking
A new FKBP51-GR-p23-Hsp902 multi-cochaperone complex identified and characterized by site-specific in-cell photocrosslinking
Taubert, M. C.; Kuehn, A.; Baischew, A.; Kaeseberg, Y.; Betschinske, J.; Hausch, F.
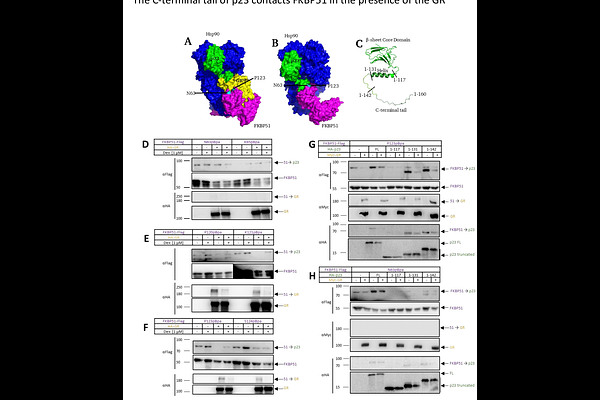
AbstractGlucocorticoid receptor (GR) activity and maturation are closely regulated by Hsp90 co-chaperones such as FKBP51, FKBP52 and p23. These co-chaperones bind to and regulate the GR, but their mutual interplay, the details of the interactions between them and their temporal dynamics are still unclear. Here we utilized UV-inducible crosslinking in living cells to map the interaction site of p23 and the GR, as well as p23 and FKBP51 at a single residue resolution. Surprisingly, we detected a novel multi co chaperone complex consisting of the GR, p23, FKBP51 and Hsp90, where both FKBP51 and p23 bind to the GR simultaneously. In this complex, FKBP51, but not the close homolog FKBP52, stabilizes the GR-p23 interaction. This is mediated in part by direct contacts between the FK1 domain of FKBP51 and the C-terminus of p23. Our findings refine the state of GR prior to activation, add a new layer of GR regulation by chaperones, provide evidence for the functional differences between FKBP51 and FKBP52, and underscore the power of photocrosslinking to functionally probe protein-protein contacts inside living cells.