Secondary structure transitions and dual PIP2 binding define cardiac KCNQ1-KCNE1 channel gating
Secondary structure transitions and dual PIP2 binding define cardiac KCNQ1-KCNE1 channel gating
Zhong, L.; Lin, X.; Cheng, X.; Wan, S.; Hua, Y.; Nan, W.; Hu, B.; Yan, Z.; Jiang, D.; Zhang, H.; Liu, F.; Xiao, C.; Zhou, Z.; Yu, H.; Ma, L.; Huang, C.; Wong, K. W.; Chung, S. K.; Shen, B.; Jiang, Z.-H.; Neher, E.; Zhu, W.; Zhang, J.; Hou, P.
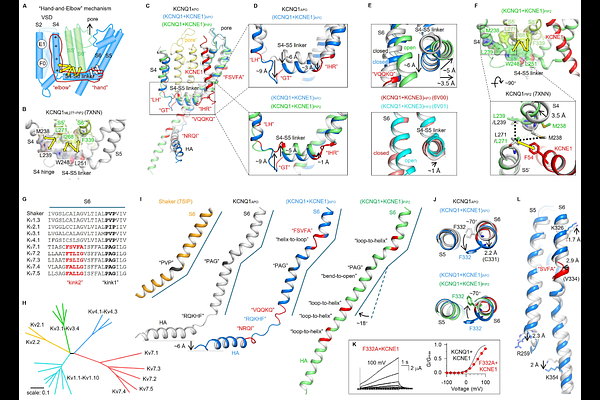
AbstractThe KCNQ1+KCNE1 potassium channel complex forms the slow delayed rectifier current (IKs) critical for cardiac repolarization. Loss-of-function variants in KCNQ1 and KCNE1 cause long QT syndrome types 1 and 5 (LQT1/LQT5), accounting for over one-third of clinical LQTS cases. Despite prior structural work on KCNQ1 and KCNQ1+KCNE3, the structural basis of KCNQ1+KCNE1 remains unresolved. Using cryo-EM and electrophysiology, we determined high-resolution (2.5-3.4 [A]) structures of human KCNQ1+KCNE1 in both closed and open states. KCNE1 occupies a pivotal position at the interface of three KCNQ1 subunits, inducing seven \"helix-to-loop\" transitions in KCNQ1 transmembrane segments. These structural rearrangements: 1) stabilize the closed pore and the conformation of the intermediate voltage-sensing domain, thereby determining channel gating, ion permeation, and single channel conductance; 2) enable a dual-PIP2 modulation mechanism, where one PIP2 occupies the canonical site, while the second PIP2 bridges the S4-S5 linker, KCNE1, and the adjacent S6\', stabilizing channel opening; 3) create a fenestration capable of binding compounds specific for KCNQ1+KCNE1 (e.g., AC-1). Together, these findings reveal a previously unrecognized large-scale secondary structural transition during ion channel gating that fine-tunes IKs function and provides a foundation for targeted LQTS therapy development.