Forecasting oncogene amplification and tumour suppressor deletion
Forecasting oncogene amplification and tumour suppressor deletion
Hernando, B.; Fernandez-Sanroman, A.; Cadiz, A.; Santamaria, P. G.; Gomez-Sanchez, D.; Escobar-Rey, M.; Chaves-Urbano, B.; Thompson, J. S.; Torres, M.; Ruiz de Garibay, G.; Adradas, V.; Alvarez, E.; The Pan Prostate Cancer Group, ; Tarabichi, M.; Lesluyes, T.; Coya, J. M.; Zugazagoitia, J.; Paz-Ares, L.; Macintyre, G.
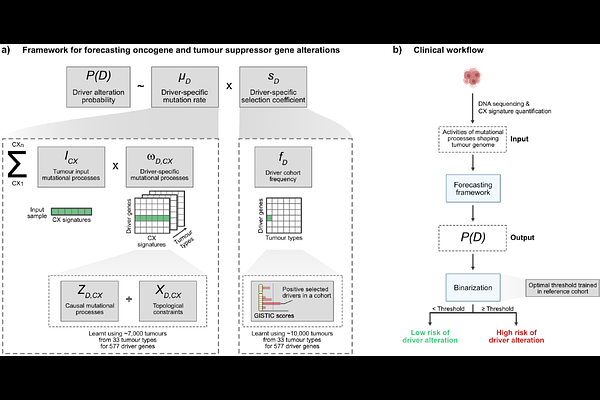
AbstractOncogene amplification and tumour suppressor deletion can drive tumour initiation, progression and treatment resistance. Detection at diagnosis often signals poor prognosis, but it can also enable opportunities for treatment with highly effective targeted therapies. Predicting the likelihood that a patient will acquire these driver alterations in the future using a genomic test represents an opportunity to realise the benefits of interventions earlier, potentially with preventative intent. Here, we present a forecasting framework that takes as input a DNA copy number profile and predicts whether the tumour will acquire an oncogene amplification or tumour suppressor deletion in the future. This framework leverages mutation rate estimates from the input tumour, alongside gene-specific selection coefficients derived from a large cohort of 7,880 tumours. We demonstrate feasibility using 7,042 single-time-point samples and longitudinally collected tumour pairs from 44 prostate and 100 lung cancers, identifying tumours that went on to acquire amplifications at a later time point with an average AUC of 0.87. We show potential clinical utility by forecasting poor prognosis in low-grade gliomas via CDK4/PDGFRA amplification or CDKN2A deletion, and osimertinib resistance in lung cancers via MET amplification. This study serves as a proof-of-concept for a new class of biomarker, wherein selective pressures and mutation-generating processes can be harnessed to anticipate future genomic alterations.