SARS-CoV-2 envelope protein induces LC3 lipidation via the V-ATPase-ATG16L1 axis
SARS-CoV-2 envelope protein induces LC3 lipidation via the V-ATPase-ATG16L1 axis
Figueras-Novoa, C.; Timimi, L. J.; Marcassa, E.; Taveira-Marques, R.; Adams, L.; Jiang, M.; Wu, M. Y.; Montaner, B.; Ng, K.; De Lorenzo, G.; Furnon, W.; Cowton, V. M.; Upfold, N.; Kassiotis, G.; Harvey, R.; Patel, A. H.; Howell, M.; Ulferts, R.; Beale, R.
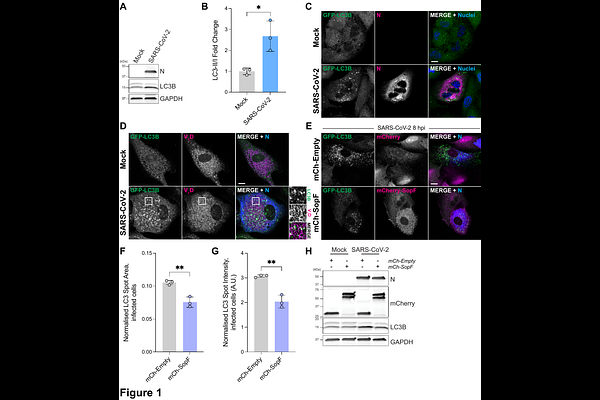
AbstractCoronaviruses encode envelope (E), a structural component of the virion that is important for assembly and egress. E has proton channel activity that prevents premature rearrangement of the spike glycoprotein as virions encounter acidic compartments as they exit the cell. How infected cells respond to this pH disruption during coronavirus infection is unknown. Here we show that SARS-CoV-2 E ion channel activity triggers the proton pump V-ATPases to recruit the ATG16L1 complex during infection. This results in ATG8 molecules such as LC3B decorating perturbed compartments. This recruitment of autophagy machinery does not inhibit viral replication, rather SARS-CoV-2 exploits this response. Inhibition of the V-ATPase/ATG16L1 interaction using the Salmonella effector SopF inhibits SARS-CoV-2 replication. Careful distinction between use of the autophagic machinery from canonical macroautophagy is required in order to better understand coronavirus replication and for rational targeting of any potential host-directed therapies.