Noninvasive ultrasound targeted modulation of calcium influx in splenic immunocytes potentiates antineoplastic immunity attenuating hepatocellular carcinoma proliferation
Noninvasive ultrasound targeted modulation of calcium influx in splenic immunocytes potentiates antineoplastic immunity attenuating hepatocellular carcinoma proliferation
Dong, W.; Wang, G.; Li, S.; Chai, Y.; Wang, Q.; Li, Y.; Fei, Q.; Zong, Y.; Geng, J.; Liu, P.; Li, Z.
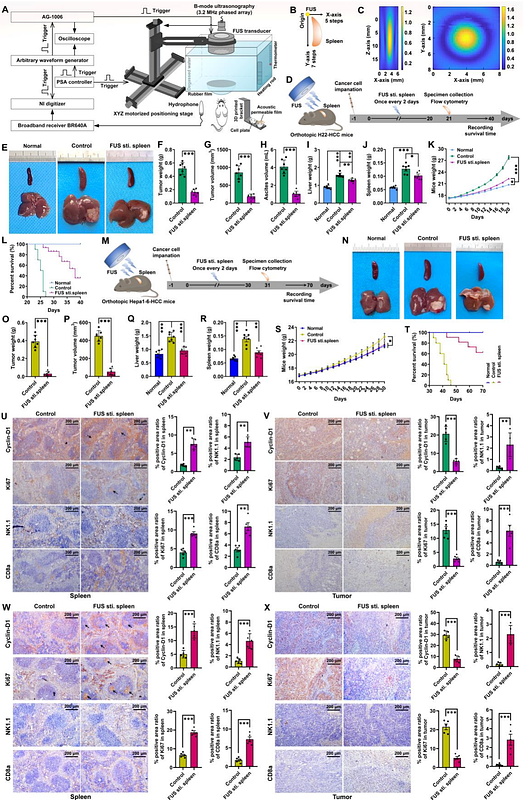
AbstractThe spleen, as the largest immune organ, plays a pivotal role in modulating immune responses, particularly in the context of carcinogenesis and tumor progression. Non-pharmacological manipulation, particularly splenic ultrasound stimulation (SUS), has demonstrated significant immunomodulatory efficacy in alleviating chronic inflammatory diseases, suggesting its potential to revitalize splenic immunocompetence suppressing tumor proliferation, yet remains underexplored. This study applied low-frequency pulsed focused ultrasound (FUS) noninvasively stimulating the spleen (FUS sti. spleen) to investigate the efficacy in enhancing antitumor immunity and suppressing hepatocellular carcinoma (HCC). The results showed that FUS sti. spleen significantly suppressed tumor proliferation, achieving a suppression rate of >70% for H22-HCC and >83% for Hepa1-6-HCC, along with significantly prolonged survival. Comprehensive flow cytometry, single-cell RNA sequencing (scRNA-seq) and cytokine analyses demonstrated that SUS profoundly reshaped the splenic and intratumoral immune landscape, specifically activating cytotoxic CD8+ T cells and NK cells while suppressing immunosuppressive cell populations. Mechanistically, FUS facilitated calcium influx in splenic immunocytes, activating multiple signaling pathways, such as TNF, NF{kappa}B, MAPK, HIF-1, and ErbB, thereby counteracting tumor-driven immunosuppressive polarization while potentiating robust immune activation that impedes malignant progression and neoplastic proliferation. Leveraging above insights, we developed spleen-targeted nanodroplets encapsulating bioavailable calcium ions (STNDs@Ca²+), which, upon FUS stimulation, undergo cavitation-mediated controlled release of Ca{superscript 2}?, further amplifying immunocyte activation and tumor suppression, achieving a remarkable H22-HCC suppression rate of over 90%. This study highlights the therapeutic potential of ultrasound-mediated splenic immunomodulation, both as a standalone intervention and in synergy with STNDs@Ca²+, as a novel and noninvasive strategy for cancer immunotherapy.