Particle-based hydrogel inks and support matrices for biofabricating structural complexity, soluble gradients, and cell-lined channels in fully granular bioprinted systems
Particle-based hydrogel inks and support matrices for biofabricating structural complexity, soluble gradients, and cell-lined channels in fully granular bioprinted systems
Tumbic, J.; Ferrarese, E.; Martinez, R.; Ackleson, T.; Delgado, D.; Highley, C. B.
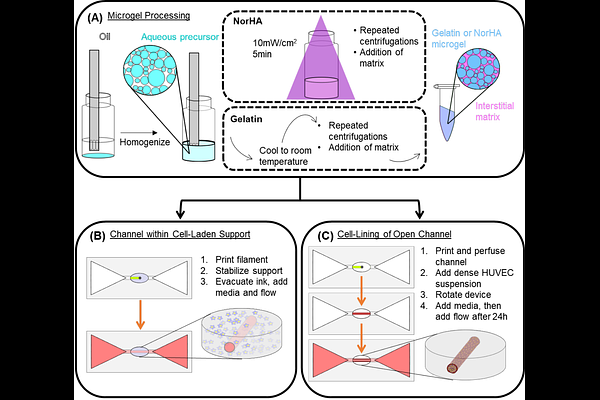
AbstractTowards achieving biomimetic complexity in biofabricated systems, an all-granular bioprinting system might use particle-based hydrogel inks to establish structures within a particle-based support matrix. In such a system, the granular support matrix can be designed to persist in the final construct and include cells incorporated prior to printing. To biofabricate complexity, bioprinting can introduce high-resolution heterogeneous structures that guide cell behaviors. The designs of the granular ink and support hydrogels are crucial to achieving complexity. High resolution structures and channels depend on small particles that flow and can be stabilized, and that can be printed and then removed, respectively. Herein, an all-granular system is described that used a granular formulation of an established, tunable hyaluronic acid-based hydrogel as the basis for a support matrix and a small particle gelatin hydrogel as an ink. Towards facilitating stabilization of the printed structure and flow during printing, the support and ink materials included soluble, interstitial components, and all exhibited yield stress behaviors characteristic of granular hydrogel systems. The support matrix\'s viscoelastic properties were dependent on intraparticle hydrogel network design, and it could be stabilized against flow by photoinitiated crosslinking. The gelatin ink could form fine filaments, as small as 100 m in testing here, and melted to leave channels within crosslinked support matrices. Channels could support flows introduced by hydrostatic pressure and could be used to rapidly transport soluble factors into the construct, which could be used to establish soluble gradients by diffusion and support cell viability. The all-granular system supported printing of complex, multimaterial structures, with feature resolution on the order of 100 m and spatial positioning on the order of 10s m. The process and materials exhibited biocompatibility with respect to cells included within the support matrix during printing or introduced into channels to begin establishing endothelialized bioprinted vessels.