OMA1-mediated cleavage of AIFM1 upon mitochondrial stress and suppression of cell growth through the control of OXPHOS activity
OMA1-mediated cleavage of AIFM1 upon mitochondrial stress and suppression of cell growth through the control of OXPHOS activity
Koshiba, T.; Nishigori, M.; Hirata, S.; Kosako, H.; Nolte, H.; Riemer, J.; Langer, T.
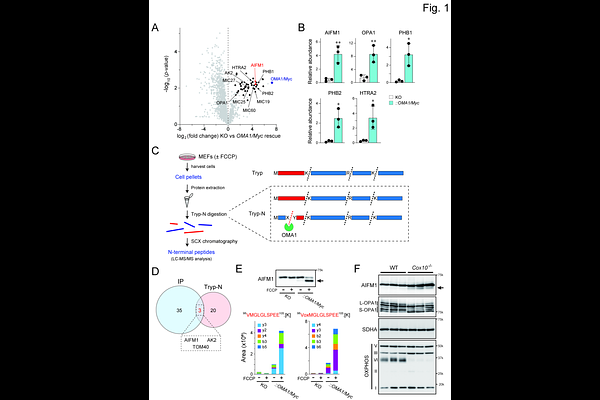
AbstractMitochondrial proteases regulate the dynamic properties of organelle morphology and ensure functional plasticity at the cellular level. The metalloprotease OMA1 mediates constitutive and stress-inducible processing of its mitochondrial substrates, but the number of functionally characterized targets remains limited. Using multiproteomic and biochemical approaches, we demonstrated that the membrane-anchored inner membrane space (IMS) protein AIFM1 serves as a mitochondrial stress-responsive substrate of OMA1. OMA1 cleaves AIFM1 in the IMS under stress conditions with a kinetically slower reaction than that of its conventional substrate, dynamin-like GTPase OPA1. Membrane dislocation of cleaved AIFM1 in mitochondria reduces its binding to subunits of the oxidative phosphorylation machinery, leading to decreased respiratory activity and ultimately impaired cell growth. Mechanistically, we revealed that AIFM1 broadly safeguards the mitochondrial proteome under steady-state conditions by mediating the import of proteins, particularly respiratory complex I subunits, via the TIM23 complex. These findings reveal a previously unrecognized role for OMA1 in integrating mitochondrial stress sensing and cellular energetics by altering the AIFM1 topology.