Transcranial ultrasound stimulation modulates neuronal membrane potentials across broad timescales in the awake mammalian brain
Transcranial ultrasound stimulation modulates neuronal membrane potentials across broad timescales in the awake mammalian brain
Bortz, E. P.; San Antonio, E.; Sherman, J.; Tseng, H.-a.; Raiff, L.; Han, X.
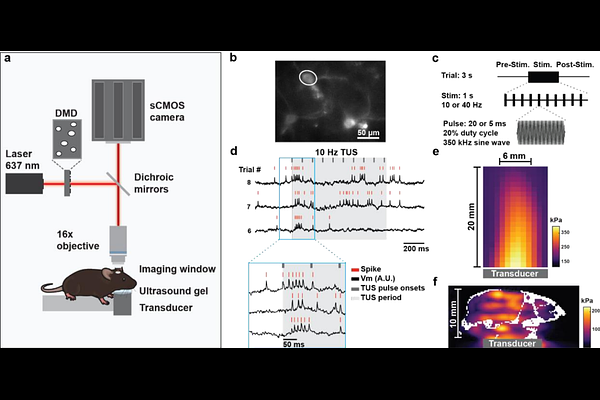
AbstractBackground: Transcranial ultrasound stimulation (TUS) offers noninvasive neuromodulation with high spatial and temporal precision, but its cellular-level effects in the awake brain remain poorly understood. Objective: We investigated how low-intensity TUS modulates membrane voltage dynamics in single cortical neurons in awake mice. Methods: Using the genetically encoded voltage indicator SomArchon, we performed high-speed kilohertz voltage imaging in awake head-fixed mice. TUS was delivered with a 0.35MHz transducer at 10 or 40Hz pulse repetition frequency with a 20% duty cycle, at intensities below the estimated threshold for auditory brainstem activation. We analyzed changes in membrane potential (Vm), spiking, and coordination across simultaneously recorded neurons. Results: TUS evoked rapid (<10ms) Vm depolarizations in 42.8% of neurons, while only 20.5% showed increased spiking, highlighting a direct effect of TUS on modulating synaptic inputs. Many neurons were entrained at both PRFs (20.8% at 10Hz; 12.7% at 40Hz) with Vm exhibiting significant phase-locking to individual TUS pulses. Vm entrainment was accompanied by increased temporal coordination across neurons and reset network synchrony. Furthermore, TUS-evoked cellular responses adapted over time, often transitioning from membrane depolarization to hyperpolarization upon repeated exposures, demonstrating prominent response depression. Conclusion: By resolving single-neuron responses, our results demonstrate that TUS directly activates individual cortical neuron with a latency shorter than 10 ms. TUS pulsed at physiologically relevant frequencies of 10 and 40 Hz robustly entrains neural dynamics, alters network coordination and evokes neuronal plasticity. These results highlight the therapeutic potential of designing TUS pulsing patterns to target specific neural circuit dynamics.