Bioinspired Oxidized mRNA Lipid Nanoparticles for Ex Vivo Engineering of Chimeric Antigen Receptor Macrophages Targeting Solid Tumors
Bioinspired Oxidized mRNA Lipid Nanoparticles for Ex Vivo Engineering of Chimeric Antigen Receptor Macrophages Targeting Solid Tumors
Mukalel, A. J.; Tylek, T.; O'Brien, E.; Frazee, C.; Geisler, H. C.; Li, J.; Thatte, A. S.; Safford, H. C.; Figueroa-Espada, C. G.; Alameh, M.-G.; Hamilton, A. G.; Mai, D.; Sheppard, N. C.; June, C. H.; Weissman, D.; Spiller, K. L.; Mitchell, M. J.
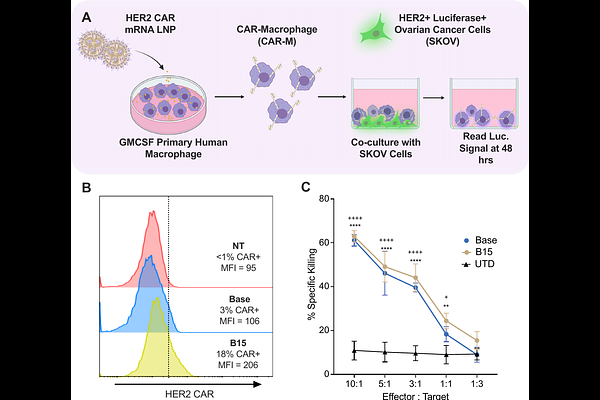
AbstractSolid tumors remain difficult to treat via conventional and novel therapeutic strategies. Immunotherapies such as chimeric antigen receptor T (CAR-T) cell therapy have been remarkably effective in treating hematological cancers, but their efficacy is limited in solid tumors. Recently, CAR macrophages (CAR-Ms) have emerged as a promising solid tumor immunotherapy, primarily for their intrinsic tumor infiltration and effector functions. However, CAR-Ms are engineered using viral transduction, which is associated with aberrant immunogenicity and toxicity. To overcome these challenges, we developed a bioinspired oxidized lipid nanoparticle (LNP) platform for mRNA-based engineering of human CAR-Ms. A library of 24 ionizable lipids was synthesized, formulated into LNPs, and screened for delivery to human macrophages. The composition of the top LNP was subsequently optimized using an orthogonal design of experiments (DoE) and physicochemical properties, such as size and mRNA encapsulation, were tuned via optimization of microfluidic mixing parameters, yielding a particle that significantly outperformed a gold standard C12-200 LNP. Utilizing small molecule and antibody inhibitors, we demonstrate that uptake of optimized LNPs into macrophages is driven by apolipoprotein E (ApoE) independent macropinocytosis, which is further supported by potent extrahepatic spleen tropism upon intravenous administration to mice. Lastly, we demonstrate the translatability of this LNP platform and utilize it to engineer functional primary human HER2-CAR-Ms ex vivo with potent antigen-specific tumor killing, validated in an ex vivo co-culture with ovarian cancer cells. This bioinspired oxidized LNP platform can potentially be utilized to engineer a range of human CAR-M immunotherapies to treat various types of solid tumors.