Activation of complement protein C3 depicts the dynamic tissue damage of the cochlea after noise trauma.
Activation of complement protein C3 depicts the dynamic tissue damage of the cochlea after noise trauma.
Guo, Z.; Seicol, B. J.; Shenouda, M.; Wang, A.; Garrity, K.; Lin, S.; Xie, R.
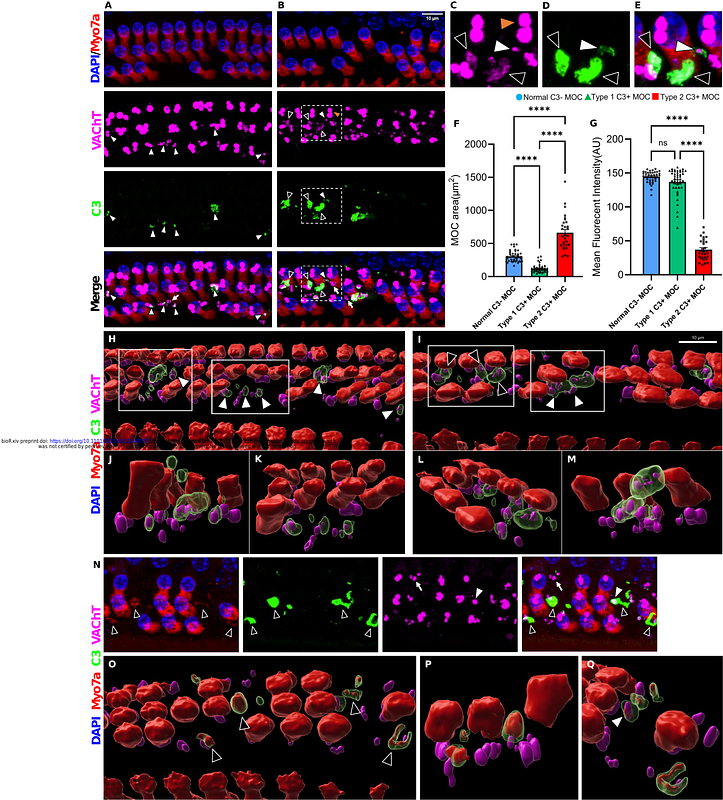
AbstractHearing loss is characterized by dysfunction and pathological changes of the cochlea, including the loss of sensory hair cells and connected synapses. Growing evidence has revealed that the immune system plays critical roles in shaping cochlear responses to tissue injury and the clearance of damaged cells under pathological conditions such as noise-induced hearing loss (NIHL). However, the underlying mechanisms that regulate this process remain unclear. As a crucial part of the innate immune system, the complement system is widely known to respond to tissue damage and help remove cellular debris. Such functions are yet to be fully explored in the auditory system, especially in the cochlea. Among various complement factors, the activation of complement protein C3 is an essential functional hub of the complement cascade, resulting in the phagocytosis of dead or dying cells. In this study, we sought to investigate whether C3 exists in the cochlea and what roles it might play upon noise injury. To test this, we studied C3 activation using immunohistochemistry in the cochleae of various mouse models that were noise exposed to an octave band noise (8-16 kHz) for two hours at 112 dB SPL. Mice were sacrificed at various post exposure times to examine cochlear pathology. We found damage dependent C3 activity in the organ of Corti in mice after noise exposure (NE), especially in damaged outer hair cell (OHC) area. Specifically, C3 opsonized damaged cellular structures include degenerating OHCs, OHC debris, ribbon synapses, and efferent synaptic boutons, and the temporal dynamics of the opsonization closely resemble the timing of cochlear tissue damages post exposure. Unexpectedly, C3-deficient mice exhibited similar ABR thresholds and OHC counts at baseline as wild type mice, as well as comparable ABR threshold shifts and OHC loss after NE. However, we observed significantly reduced MOC terminal bouton degeneration in C3-deficient mice upon 112 dB SPL noise injury, indicating that C3 does modulate tissue damage and clearance, at least at the MOC terminals. Our results suggest that complement C3 is dynamically activated in the cochlea upon noise injury, which may play significant roles in damage recognition and further immune recruitment during the development of NIHL.