Targeted degradation of Drosophila FOG homolog U-shaped mimics macrophage transdifferentiation in S2 cells
Targeted degradation of Drosophila FOG homolog U-shaped mimics macrophage transdifferentiation in S2 cells
Trummel, D.; Lenz, J.; Milani, W.; Joost, L. S.; tom Dieck, L. N.; Bogdan, S.; Brehm, A.
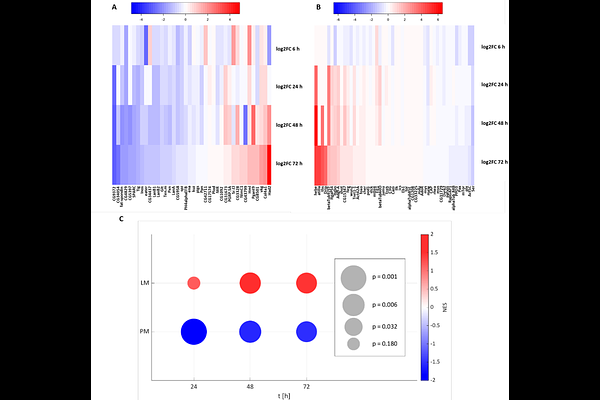
AbstractThe highly plastic cellular component of the Drosophila immune system consists of three main blood cell types - plasmatocytes, crystal cells and lamellocytes - that together allow effective responses to various insults. Infection with parasitic wasp eggs results in a rapid increase in highly specialized lamellocytes that are generated by differentiation from hemocyte precursors as well as by transdifferentiation from plasmatocytes. How differentiation and transdifferentiation are regulated at the molecular level is not well understood. Here, we show that inducible degradation of the Friend of GATA (FOG) homolog U-shaped (Ush) in the plasmatocyte-like S2 cell line results in downregulation of plasmatocyte marker genes and subsequent upregulation of lamellocyte marker genes. This transcriptional shift is accompanied by morphological and functional changes consistent with lamellocyte cell identity, including increased cell spreading and adhesion, driven by enhanced integrin expression associated with increased focal adhesions. Our findings demonstrate that targeted Ush depletion is sufficient to reprogramme plasmatocyte-like S2 cells toward a lamellocyte-like state, thereby providing the first in vitro model for studying processes involved in macrophage transdifferentiation.