Scavenger Receptor class F member 2 is an intracellular receptor for Hepatitis B virus
Scavenger Receptor class F member 2 is an intracellular receptor for Hepatitis B virus
Li, C.; Wang, Y.; Xiong, R.; Li, Q.; Zhou, Z.; Liu, Q.; Qi, Y.; Gao, Z.; Xu, G.; Fang, L.; Sun, Y.; Farzan, M.; Choe, H.; Sui, J.; Li, W.
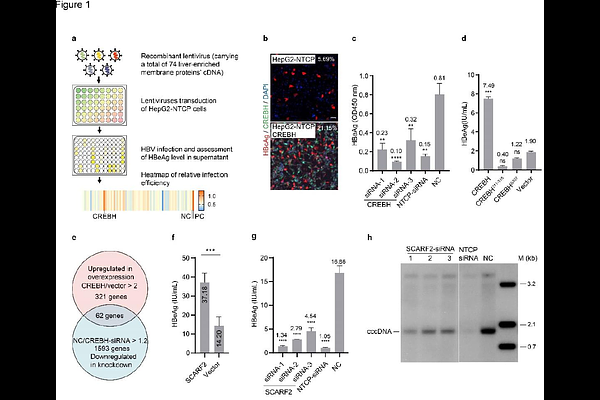
AbstractHepatitis B virus (HBV) infects hepatocytes by specific binding to the cell-surface receptor -sodium taurocholate cotransporting polypeptide (NTCP)-through the preS1 region of its large envelop protein, followed by a less well understood transport process across the cytoplasm to the nucleus. Here, we report that scavenger receptor class F member 2 (SCARF2), a single-pass transmembrane protein, functions as an intracellular receptor for HBV. SCARF2 binds to a preS1 region downstream of the NTCP binding site through its N-terminal EGF-like domains 4-6, and its proline-rich C-terminal domain also plays an indispensable role in the infection. The internalized HBV virions are transported to the cytoplasmic side of nuclear pore complexes (NPCs) within the SCARF2-containing endosomes. HBV capsid release from the endosomal vesicles is significantly impaired by knockdown of SCARF2. These results suggest a model that SCARF2 conveys HBV to the periphery of NPCs and ultimately leads to viral capsid release for nuclear entry.