mRNA interactions promote cotranslational association of heteromeric membrane proteins
mRNA interactions promote cotranslational association of heteromeric membrane proteins
Flores-Aldama, L.; Hoth, A. S.; de Carvalho, L.; Seim, I.; Gladfelter, A. S.; Robertson, G.
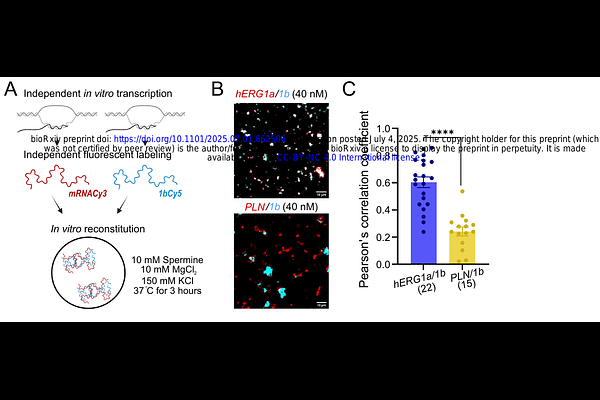
AbstractHeteromeric membrane proteins play crucial physiological roles, yet how they are formed remains poorly understood. Heteromeric hERG1a/1b ion channels, essential for maintaining normal cardiac rhythm, assemble via cotranslational association of their encoding mRNAs. We hypothesized that direct hERG1a and 1b mRNA interactions facilitate this process. Using fluorescence colocalization and free energy of binding predictions, we found that hERG1a and 1b mRNAs form specific heterotypic condensates in vitro, suggesting direct interactions. When hERG1a mRNA was altered by synonymous mutations predicted to reduce its structural diversity and ability to interact with other mRNAs, overlap with hERG1b was dramatically diminished both in vitro and in cells, indicating weakened interactions. Reducing hERG1a structural diversity also influenced its translational complexes, defined by overlapping between fluorescently labeled mRNA and encoded protein (centroids within 400 nm). Whereas most of the wild-type hERG1a mRNA translates within heterotypic complexes, likely reflecting the biogenesis of hERG1a/1b heteromeric assemblies, reducing hERG1a structural diversity yielded more homotypic translational condensates and fewer hERG1a/1b heterotypic ones. This result suggests that the strength of mRNA interactions impact ion channel biogenesis. Further analysis of the heterotypic translational complexes revealed two distinct classes: a) simultaneous translation of both subunits and b) sequential association of fully translated hERG1b with translating hERG1a. Notably, reducing hERG1a structural diversity and interactions with 1b shifted translation toward the sequential mode. These findings identify a new role of mRNA sequence, structure, and interactions in orchestrating the cotranslational association of important heteromeric membrane proteins.