In vivo CRISPR screening identifies regulators of hyperplastic and hypertrophic adipose remodelling in zebrafish
In vivo CRISPR screening identifies regulators of hyperplastic and hypertrophic adipose remodelling in zebrafish
Wafer, R.; Tandon, P.; Minchin, J. E.
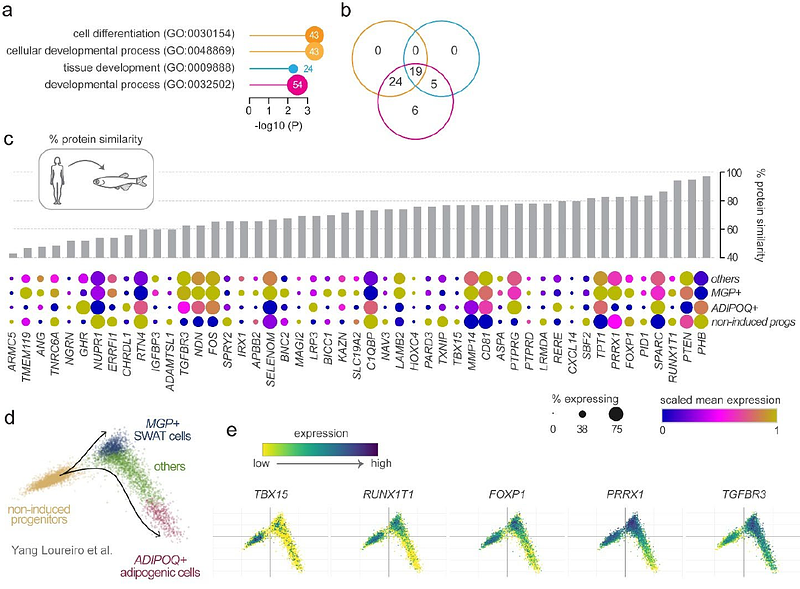
AbstractAdipose tissues exhibit a remarkable capacity to expand, regress, and remodel in response to energy status. The cellular mechanisms underlying adipose remodelling are central to metabolic health. Hypertrophic remodelling, characterised by the enlargement of existing adipocytes, is associated with insulin resistance, type 2 diabetes, and cardiovascular disease. In contrast, hyperplastic remodelling, in which new adipocytes are generated, is linked to improved metabolic outcomes. Despite its clinical importance, the regulation of hypertrophic and hyperplastic adipose remodelling remains poorly understood. In this study, we first leveraged human genetic and transcriptomic data to identify candidate genes involved in adipose remodelling. We then developed a quantitative imaging pipeline to assess hyperplastic and hypertrophic morphology in zebrafish subcutaneous adipose tissue, and applied it in an F0 CRISPR mutagenesis screen targeting 25 candidate genes. This screen identified six genes that significantly altered adipose morphology; including Sushi Repeat Containing Protein (Srpx), a gene with previously unknown roles in adipose. Among the identified genes, foxp1b mutants were notable for inducing hypertrophic morphology. To investigate further, we generated stable loss-of-function alleles for both zebrafish foxp1 genes. We found that foxp1b mutants display a developmental bias towards hypertrophic adipose growth but fail to undergo further hypertrophic remodelling in response to a high-fat diet - suggesting that early developmental patterning constrains later adaptability to diet. Together, these findings establish a scalable and tractable in vivo screening platform for identifying regulators of adipose remodelling, and reveal a potential developmental influence on the capacity for diet-induced adipose expansion.