Discovery of excited state proton transfer in flavin-based fluorescent protein with large Stokes shift
Discovery of excited state proton transfer in flavin-based fluorescent protein with large Stokes shift
Nikolaev, A.; Markeeva, E.; Maksimov, E. G.; Yudenko, A.; Tropina, E. V.; Boldyrev, K. N.; Nikolaeva, Y. S.; Cherepanov, D. A.; Kovalev, K.; Yang, Y.; Borshchevskiy, V. I.; Kuznetsova, E.; Kuzmin, A.; Semenov, O.; Remeeva, A.; Gushchin, I.
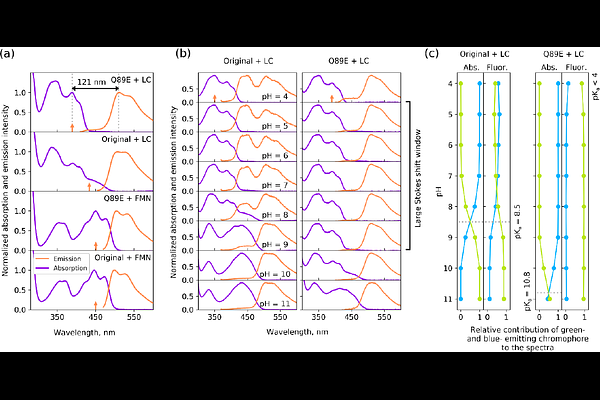
AbstractFlavin-binding proteins (flavoproteins) are widespread in nature, revealing versatile oxidation-reduction reactions and photochemistry. Flavoproteins derived from LOV domains are used for engineering of light-responsive tools in optogenetics, as well as fluorescent markers and photogenerators of reactive oxygen species. Despite extensive efforts, all currently used LOV-derived proteins have similar absorption spectra with maxima around 275, 350-375, and 450-485 nm. Here, we describe the discovery of a large Stokes shift flavin-based fluorescent protein, LSSFbFP, which can be obtained in vivo and in vitro, with absorption maxima at 340-350 and 395-405 nm. Fluorescence emission of LSSFbFP mirrors that of classical FbFPs with the maximum at ~500 nm. We show that the protein binds lumichrome as the chromophore and use low temperature and time-resolved spectroscopy, X-ray crystallography and modeling to prove that the apparent Stokes shift of LSSFbFP occurs due to excited state proton transfer from lumichrome\'s N1. Our findings expand the range of photochemical phenomena observed in flavoproteins and pave the way for engineering of new flavin-based molecular instruments.