Membrane phosphoinositides allosterically tune β-arrestin dynamics to facilitate GPCR core engagement
Membrane phosphoinositides allosterically tune β-arrestin dynamics to facilitate GPCR core engagement
Janetzko, J.; Deutsch, J.; Shi, Y.; Siepe, D. H.; Masureel, M.; Liu, W.; Viner, R.; Inoue, A.; Kobilka, B. K.; Shivnaraine, R. V.
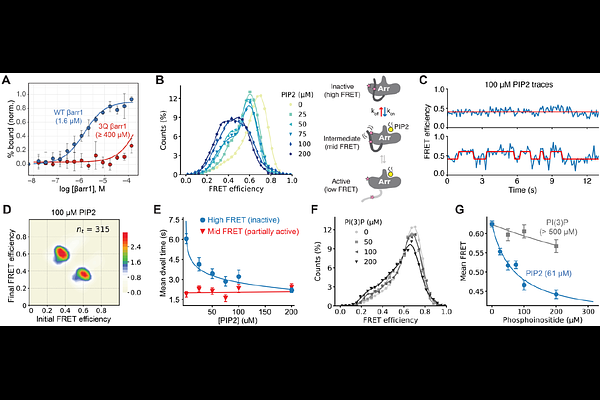
AbstractArrestin proteins bind active G protein-coupled receptors (GPCRs) through coordinated protein-protein, protein-phosphate, and protein-lipid interactions to attenuate G protein signaling and promote GPCR internalization and trafficking. While there are hundreds of diverse GPCRs, only two {beta}-arrestin isoforms ({beta}arrs) must recognize and engage this wide range of receptors with varied phosphorylation patterns. Traditional models suggest that {beta}arr activation requires displacement of its autoinhibitory C-tail by a phosphorylated GPCR C-terminus; however, this paradigm fails to explain how minimally phosphorylated GPCRs still complex with {beta}arrs. Using single-molecule Förster resonance energy transfer imaging and hydrogen-deuterium exchange mass spectrometry, we observe basal dynamics in which the {beta}arr1 C-tail spontaneously releases from the N-domain, transiently adopting an active conformation that can facilitate binding of the phosphorylated GPCR C-terminus. We further demonstrate the importance of an intermediate state of {beta}arr1 arising from spontaneous C-tail release stabilized by the membrane phosphoinositide PI(4,5)P2. Both PI(4,5)P2 and mutations in the proximal or middle regions of the C-tail shift {beta}arr1 towards a partially released state, revealing an allosteric connection that informs a refined model for {beta}arr activation. In this model, membrane engagement conformationally primes {beta}arrs prior to receptor binding, thereby explaining how {beta}arrs are recruited by diverse GPCRs, even those with limited C-terminal phosphorylation.