Allosteric Regulation of RNA Affinity by Motif V-VI Coupling in West Nile Virus NS3 Helicase
Allosteric Regulation of RNA Affinity by Motif V-VI Coupling in West Nile Virus NS3 Helicase
Roy, P.; McCullagh, M.
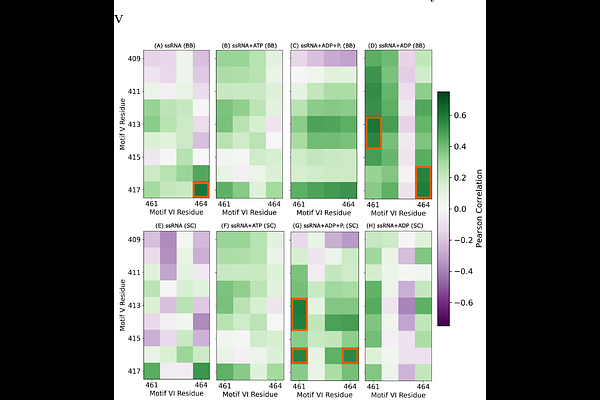
AbstractThe rise of flaviviral diseases, including West Nile virus (WNV), presents a growing threat to global public health and underscores the urgent need for new therapeutic strategies. The non-structural protein 3 helicase (NS3h) of the Orthoflavivirus genus, including WNV, is essential for viral replication and a promising antiviral target. Previously [Roy et al., Nucleic Acids Research, 52, 13, 2024, 7447-7464], we showed that the motif VI loop (VIL) in WNV NS3h functions as a nucleotide valve, regulating ADP affinity during hydrolysis. In this study, we uncover an ATP-dependent coupling between nucleotide affinity at motif VIL and RNA affinity at motifs IVa and V, suggesting a coordinated mechanism of ssRNA translocation. Using microsecond-scale all-atom molecular dynamics simulations of hydrolysis-cycle intermediates, we find that key VIL residues (R461, R464) correlate strongly with RNA phosphate affinity of motif V. Structural analyses reveal an ATP-sensitive interaction between E413 (motif V) and R461 (motif VIL) that modulates the conformation of the motif V 310-helix, thereby influencing RNA binding. This dynamic interaction is lost in catalytically deficient VIL mutants, which have been experimentally shown to impair hydrolysis and attenuate viral replication. These findings provide mechanistic insights into NS3h function and identify new opportunities for structure-based antiviral design.