In vivo RUBISCO activity in Synechocystis is regulated by RuBP availability
In vivo RUBISCO activity in Synechocystis is regulated by RuBP availability
Wittemeier, L.; Arrivault, S.; Neumann, N.; Macek, B.; Schmidt, N.; Hagemann, M.; Kopka, J.
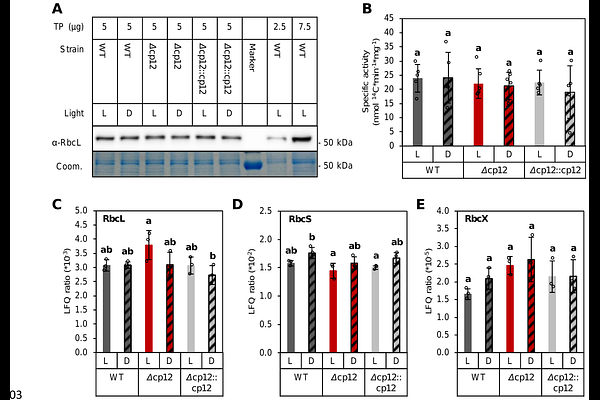
AbstractRibulose-1,5-bisphosphate carboxylase/oxygenase (RUBISCO) is the main CO2-fixing enzyme on earth and entry point of carbon into the Calvin-Benson-Bassham cycle. Fueled by photosynthesis, C-assimilation by RUBISCO must be tightly controlled. RUBISCO regulation upon transition from light to darkness is not fully understood in the cyanobacterium Synechocystis sp. PCC 6803 (Synechocystis). Synechocystis does not have a RUBISCO activase that regulates RUBISCO activity in vascular plants by removing intrinsic sugar phosphate inhibitors from its active site. Instead, the regulatory CP12 protein of Synechocystis inactivates glyceraldehyde 3-phosphate dehydrogenase (GAPDH2) and phosphoribulokinase (PRK) during darkness. This mechanism indicates metabolic regulation of RUBISCO. We investigated C-assimilation in vivo at the transition to darkness by dynamic 13CO2 labeling experiments. We monitored RUBISCO activity by 13C-incorporation into 1-C position of 3PGA. Other than the wild type, the{Delta} cp12 mutant continued to assimilate 13CO2 into 3PGA in darkness. RUBISCO abundances and specific activities were not altered in{Delta} cp12 and upon light to dark transition. CP12 was required to shut down the CBB cycle during the night. Complementation of{Delta} cp12 by native CP12 ({Delta}cp12::cp12) and CP12 with mutated conserved cysteines in its GAPDH2- and PRK-binding domains ({Delta}cp12::cp12{Delta}Cys) showed that both native binding domains are required to fully inactivate the CBB cycle in the night. RuBP levels were highly elevated in{Delta} cp12 upon transition to darkness. Complementation with mutated and native CP12 variants gradually reduced RuBP to wild type levels and revealed highly significant correlation between RuBP concentration and the time-shifted 13C-uptake into 3PGA. We propose that RUBISCO activity in Synechocystis at day-night transition is regulated through depletion and blocked regeneration of RuBP. 13C-positional analyses of aspartate suggest regeneration of RuBP in{Delta} cp12 via dysregulated gluconeogenesis and the oxidative pentose phosphate path. We demonstrate that RUBISCO activity of Synechocystis is present throughout diurnal growth and depends on the availability of its substrate.