Scaling relations of multi-component phase coexistence boundaries
Scaling relations of multi-component phase coexistence boundaries
Qian, D.; Acker, J.; Scrutton, R.; Fischer, C. M.; Qamar, S.; Sneideris, T.; Borodavka, A.; Knowles, T.
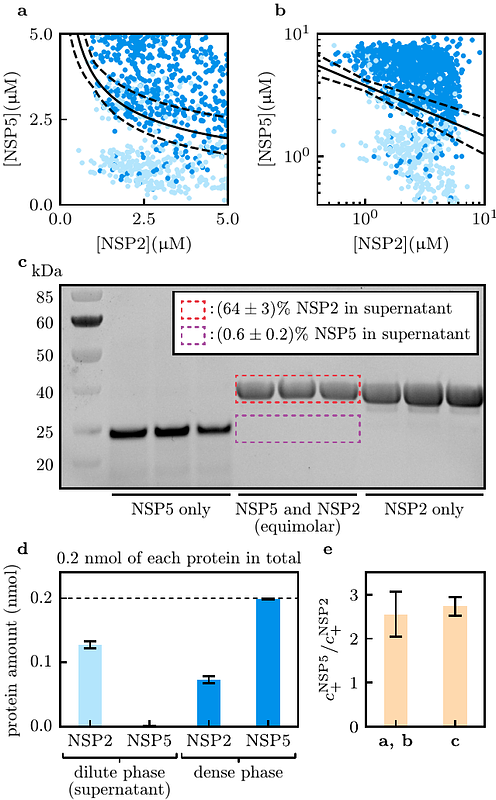
AbstractMulticomponent phase-separating molecular systems play a key role as membraneless organelles in living cells. Phase diagrams are indispensable for probing the concentration-dependent behaviour of these condensates, yet their interpretation has remained largely qualitative due to the challenges of modelling complex, multicomponent interactions. Here, we present a generic framework for quantitative analysis of phase diagrams. We derive an analytical expression for the phase boundary normal vector, which reveals two distinct scaling regimes: one for associative phase separation, characterised by a power-law scaling analogous to mass-action kinetics that allows for direct extraction of dense phase solute molar ratios, and another for condensate dissolution, where an exponential scaling quantifies three-body repulsion effects triggered by the addition of a new component. We demonstrate the practical utility of our framework by applying it to a range of experimentally measured phase diagrams, including those for proteins such as NSP5, NSP2, FUS, Whi3, G3BP1, and antimicrobial peptides. Collectively, our work not only provides a clear quantitative interpretation of phase diagrams but also opens new avenues for the rigorous characterisation of multicomponent phase-separating systems.