Quantifying the Cooperativity of Backbone Hydrogen Bonding
Quantifying the Cooperativity of Backbone Hydrogen Bonding
Xu, Y.; Huang, J.
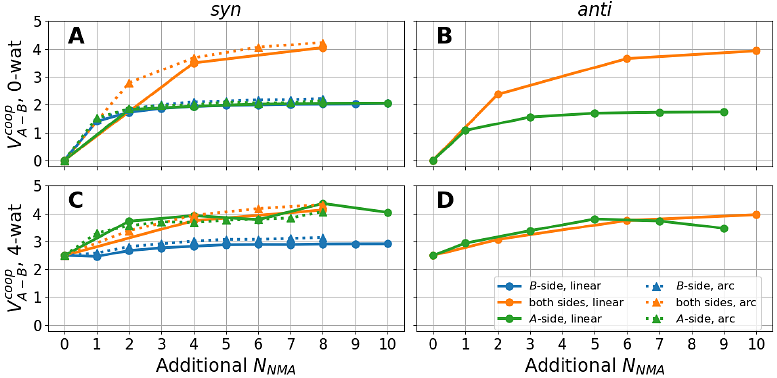
AbstractThe hydrogen bonds (H-bonds) between backbone amide and carbonyl groups are fundamental to the stability, structure, and dynamics of proteins. A key feature of such hydrogen bonding interactions is that multiple H-bonds can enhance each other when aligned, as such in the -helix or {beta}-sheet secondary structures. To better understand this cooperative effect, we propose a new physical quantity to evaluate the cooperativity of intermolecular interactions. Using H-bond aligned N-methylacetamide molecules as the model system, we assess the cooperativity of protein backbone hydrogen bonds using quantum chemistry (QM) calculations at the MP2/aug-cc-pVTZ level, revealing cooperative energies ranging from 2 to 4.3 kcal/mol. A set of protein force fields was benchmarked against QM results. While the additive force field yielded null cooperativity, polarizable force fields, including the Drude and AMOEBA protein force fields, have been found to reproduce the trend of QM results, albeit with smaller magnitude. This work demonstrates the theoretical utility of the proposed formula for quantifying cooperativity and its relevance in force field parameterization. Incorporating cooperative energy into polarizable models presents a pathway to achieving more accurate simulations of biomolecular systems.