Nephron segmentation and patterning in kidney organoids can be modulated by distinct FGF subfamily members
Nephron segmentation and patterning in kidney organoids can be modulated by distinct FGF subfamily members
Zylberberg, A. K.; Scully, E. I.; Er, P. X.; Baric, H.; Scurr, M.; Xie, M.; Peiris, T.; Howden, S. E.; Lawlor, K. T.; Little, M. H.
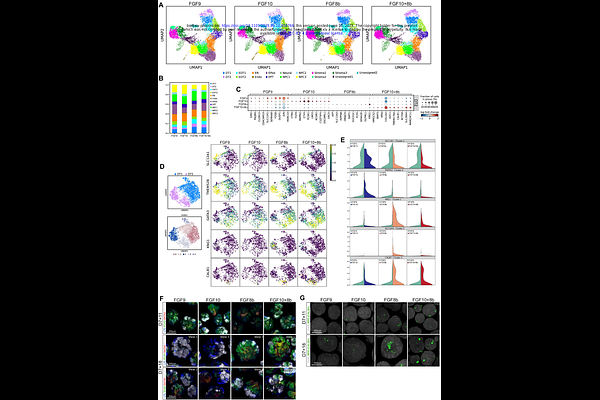
AbstractHuman pluripotent stem cell-derived kidney organoids replicate embryonic nephrogenesis in a 3D-culture system. Recent advances suggest that refining the culture environment to replicate spatiotemporal cues present during embryonic organogenesis improves patterning. Here, this paradigm was applied to FGF signalling, a key regulator of embryonic nephron progenitor maintenance, nephrogenesis and ureteric branching. Both FGF8b and FGF10 signalling is sufficient to support nephrogenesis, with each having distinct effects on nephron patterning. FGF10 enhanced the initial WT1+ mesenchymal population, leading to proximally biased nephrons, while FGF8b biased toward early distal patterning, leading to the formation of cells with connecting segment identity. The addition of both FGF8b and FGF10 together had an additive effect, leading to a balance of proximal and distal patterning. This differential patterning was retained in tissue transplanted under the murine renal capsule, with FGF8b-treated organoids displaying increased distal/connecting segments. These findings highlight plasticity during organoid nephrogenesis that can be modulated by FGF signalling and identify an approach to refine nephrogenesis toward key cell types.