Multiple weak brakes act in concert to regulate STIM1 and control store-operated calcium entry
Multiple weak brakes act in concert to regulate STIM1 and control store-operated calcium entry
Qiu, R.; Lewis, R. S.
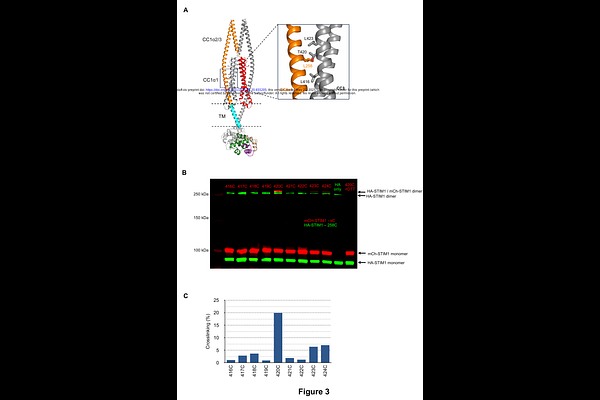
AbstractThe ER Ca2+ sensor STIM1 evokes store-operated Ca2+ entry through the interaction of its cytosolic CRAC activation domain (CAD) with the plasma membrane Ca2+ channel Orai1 following ER Ca2+ depletion. To avoid pathophysiological effects, STIM1 must respond precisely and reversibly to changes in ER Ca2+ content while maintaining a low basal activity under resting, ER-replete conditions. Here we combine single-molecule FRET measurements of full-length dimeric STIM1 in lipid membranes with an AlphaFold2 structural model to describe the structure and regulation of the resting state. We show that STIM1 activity is controlled by the combined operation of four relatively weak restraints, or brakes. The Ca2+-bound EF-SAM luminal domain acts as a steric restraint to inhibit spontaneous activity. In the cytosolic region, the domain-swapped interaction and alignment of CC11 with CC3 of CAD positions the apex of CAD next to the ER membrane, where electrostatic lipid-protein interactions further stabilize the inactive conformation. A fourth brake is created by hydrophobic and electrostatic interactions of the two CC12/3 domains attached to the base of CAD. Disruption of any one of these brakes leads to STIM1 activation, showing that the concerted action of these relatively weak restraints is required to prevent spontaneous activity in resting cells with full ER Ca2+ stores, while enabling reversible activation in response to changes in store content.