PD-L1 ligation on NK cells induces a metabolic shift from glycolysis to fatty acid oxidation, enhancing tumor infiltration and control
PD-L1 ligation on NK cells induces a metabolic shift from glycolysis to fatty acid oxidation, enhancing tumor infiltration and control
Srpan, K.; Lupo, K.; Sottile, R.; Scarno, G.; Lawry, C.; Kolk, G.; Shaffer, B.; Patel, S. G.; Al-Niaimi, A.; Lau, C. M.; Sun, J.; Hsu, K. C.
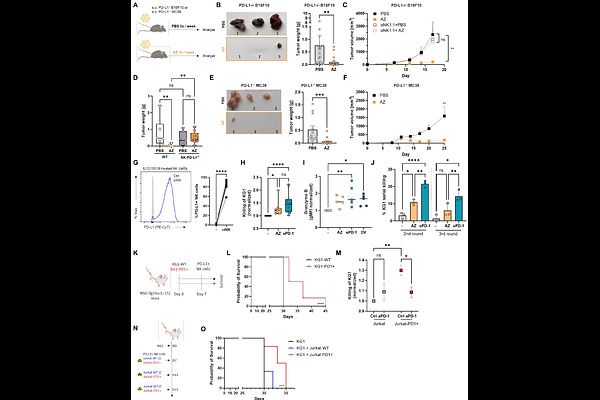
AbstractPD-L1 blockade benefits even PD-L1-negative tumors, suggesting that non-tumor cells contribute to PD-L1 expression. Natural killer (NK) cells, vital mediators of innate immunity, vigorously express PD-L1 upon activation. We demonstrate that the ligation of PD-L1 on circulating and tumor-infiltrating NK cells with the therapeutic anti-PD-L1 antibody atezolizumab, soluble PD-1, or PD-1+ cells enhances NK cell-mediated tumor clearance via changes in metabolism, adhesion, and migration. PD-L1 engagement increases NK cell tumor infiltration via the CXCR3 pathway and cytoskeletal remodeling, supported by a metabolic shift from glycolysis to fatty acid oxidation (FAO). Loss of a key FAO enzyme, CPT1A, in NK cells abrogates the PD-L1-mediated anti-tumor effect, supporting a critical role for FAO in enhanced NK cell killing. The PD-L1-triggered shift away from glycolysis permits NK cells to remain highly effective at tumor killing in glucose-restricted TME. Taken together, PD-L1 ligation enhances NK cell cytotoxicity and tumor infiltration and contributes to NK resilience in challenging TME conditions, resulting in a more effective anti-tumor immunity.