Structural insights into allosteric mechanism of glycine transporter-mediated analgesia
Structural insights into allosteric mechanism of glycine transporter-mediated analgesia
Cantwell Chater, R. P.; Peiser-Oliver, J.; Pati, T. K.; Quinn, A. S.; Lotsaris, I.; Frangos, Z. J.; Tischer, A. E.; Williams-Noonan, B. J.; O'Mara, M. L.; Michaelides, M.; Mohammadi, S. A.; Cioffi, C. L.; Vandenberg, R. J.; Shahsavar, A.
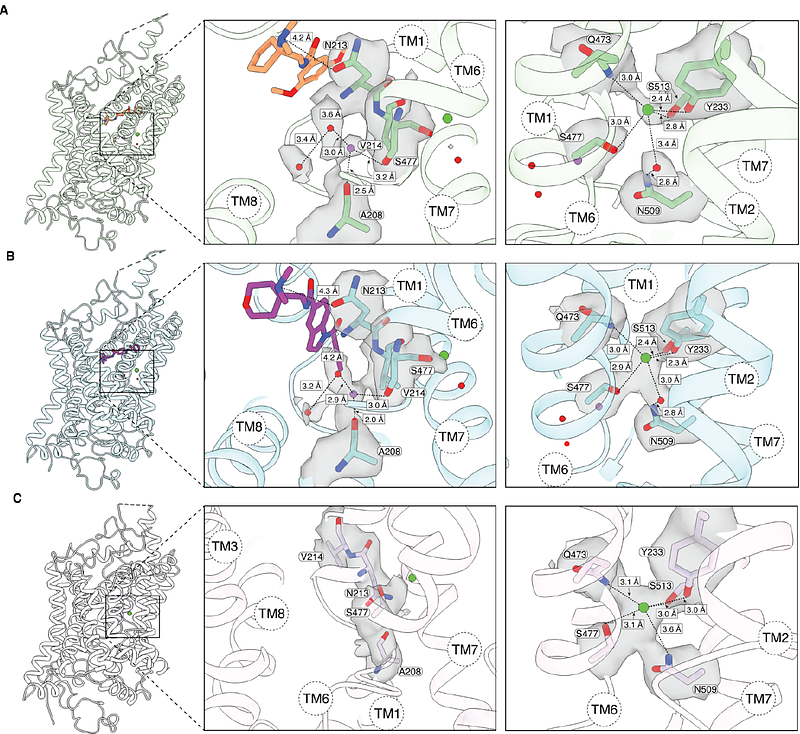
AbstractChronic neuropathic pain, caused by nerve damage or disease, affects 10% of the population with rising incidence. The burden is exacerbated by ineffective treatments and overreliance on opioids. Neuronal glycine transporter, GlyT2, represents a promising target to restore disrupted inhibitory glycinergic neurotransmission in neuropathic pain. However, most GlyT2 inhibitors have not progressed to clinical use because of significant side effects, partly from irreversible inhibition at analgesic doses. Here, we report cryo EM structures of human GlyT2 bound to the potent pseudo irreversible inhibitor ORG25543, a reversible analogue RPI-GLYT2-82, and in substrate-free state. Both inhibitors bind an extracellular allosteric site, locking the transporter in an outward open conformation, whereas the substrate-free GlyT2 adopts an inward-open conformation. We demonstrate that RPI GLYT2 82 has a faster off-rate, providing analgesia in mouse neuropathic pain models, with no observed mechanism-based side-effects or addiction liability. Our data provide a model for allosteric inhibition, enabling structure-based design of new non-opioid analgesics.