Metabolic Strategies That Enable Oral Commensal Persistence in a Lower Airway Environment
Metabolic Strategies That Enable Oral Commensal Persistence in a Lower Airway Environment
Toney, A. M.; Koirala, J.; Stacy, A.
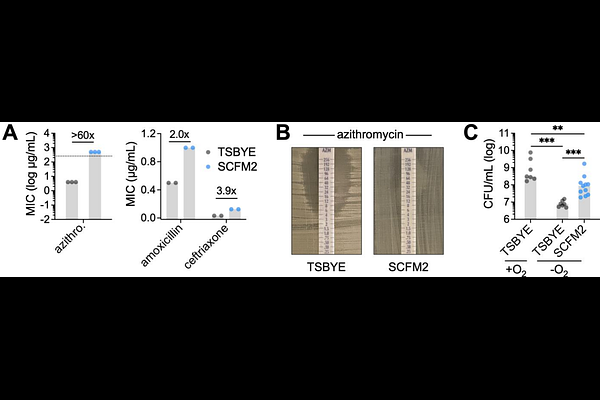
AbstractOral microbiota are increasingly implicated in chronic inflammatory diseases beyond the mouth, including bronchiectasis, a condition marked by persistent airway inflammation, mucus accumulation, and limited therapeutic options. Among these microbes, commensal Neisseria, typically considered health-associated in the upper airway, are emerging as opportunistic colonists of the inflamed lower airway. However, mechanisms supporting their persistence in this hostile environment, characterized by antibiotic pressure, nutrient limitation, and interspecies competition, remain poorly defined. Here, we show that the prevalent oral commensal Neisseria mucosa exhibits markedly enhanced antibiotic resistance and anoxic growth in a synthetic medium (SCFM2) that mimics sputum from individuals with bronchiectasis. Using genome-wide transposon sequencing (Tn-seq), we identified key genetic determinants of N. mucosa fitness, including pathways for L-lactate catabolism and pyrimidine biosynthesis. Notably, nitrate respiration was also essential for growth, linking use of this inflammation-associated electron acceptor to fitness under oxygen-limited, sputum-like conditions. Loss of nitrate reductase impaired anoxic L-lactate catabolism, reduced competitive fitness against Pseudomonas aeruginosa, and abrogated growth in SCFM2, as well as human saliva--a relevant, nitrate-rich nutrient source in the oral cavity. Furthermore, chemical inhibition of nitrate reductase using tungstate suppressed growth of both N. mucosa and P. aeruginosa in SCFM2, but not in standard media, revealing a context-specific metabolic vulnerability of nitrate-respiring airway pathogens. These findings suggest that inflammation-compatible traits like nitrate respiration, while supporting oral commensalism, may also drive opportunistic expansion in the inflamed airway. Targeting such pathways could offer a non-antibiotic approach to limit oral bacterial persistence in bronchiectasis and other chronic diseases.