Epigenetic Regulation of Chondrogenesis: JMJD3 and UTX as Key Targets for Gene-Modified Mesenchymal Stem Cell Therapy in Cartilage Tissue Engineering
Epigenetic Regulation of Chondrogenesis: JMJD3 and UTX as Key Targets for Gene-Modified Mesenchymal Stem Cell Therapy in Cartilage Tissue Engineering
Allas, L.; Aury-Landas, J.; Rochoux, Q.; Julien, A.; Lhuissier, E.; Lente, M.; Brochard, S.; Veyssiere, A.; Boumediene, K.; Bauge, C.
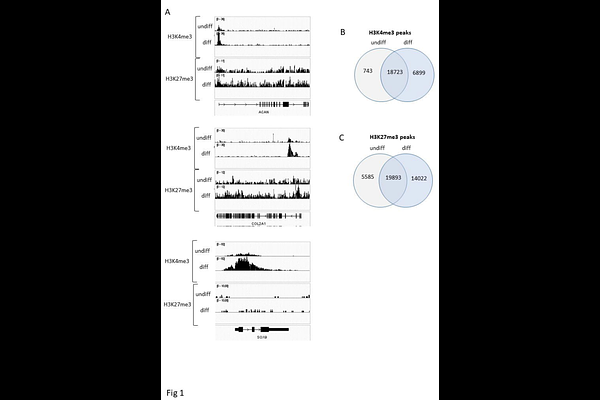
AbstractOsteoarthritis is a major cause of disability in older adults, and among the promising therapeutic strategies, cartilage tissue engineering shows great potential. Histone methylation plays a critical role in cartilage development, making it an appealing target for improving cartilage engineering protocols. In this study, we evaluated the roles of JMJD3 (KDM6B) and UTX (KDM6A), both demethylases of histone H3 at lysine 27 (H3K27), in chondrogenesis and their application in gene-modified mesenchymal stem cell therapy for cartilage tissue engineering. Using high-throughput analyses such as ChIP-Seq and whole-transcriptome microarray, we explored the functions of JMJD3 and UTX in human bone marrow-derived mesenchymal stem cells (hBM-MSC) undergoing chondrogenesis. We investigated the impact of inhibiting JMJD3 and UTX with the pharmacological inhibitor GSK-J4 or using siRNA. Additionally, the effects of transiently transfecting JMJD3 or UTX expression vectors were assessed both in vitro and in vivo, following the implantation of hBM-MSC embedded in alginate in nude mice. Our findings revealed that JMJD3 is specifically upregulated during chondrogenesis in hBM-MSC, and is crucial for this differentiation process. In contrast, UTX was found to be dispensable for chondrogenesis. Nevertheless, both JMJD3 and UTX share the ability to demethylate similar gene loci, thereby promoting the expression of chondrogenic signature genes, which suggests functional redundancy. Notably, the genes encoding these H3K27me3 demethylases emerge as strong candidates for enhancing gene-modified mesenchymal stem cell therapy for cartilage tissue engineering, as their overexpression during chondrogenesis significantly increased the formation of thicker cartilage discs enriched with type II collagen. In conclusion, this study provides important insights into the epigenetic regulation of chondrogenesis, especially regarding the role of H3K27me3 demethylases. We demonstrate that, although JMJD3 and UTX have overlapping targets, only JMJD3 is critical for the chondrogenesis process. Additionally, the findings emphasize the potential of transient JMJD3 transduction, along with a lesser emphasis on UTX, as effective strategies for improving gene-modified mesenchymal stem cell therapy in cartilage tissue engineering.