Imaging CRISPR-Edited CAR-T Cell Therapies with Optical and Positron Emission Tomography Reporters
Imaging CRISPR-Edited CAR-T Cell Therapies with Optical and Positron Emission Tomography Reporters
Sanchez-Pupo, R. E.; Kelly, J. J.; Shalaby, N.; Xia, Y.; Martinez Santiesteban, F. M.; Lau, J.; Verriet, I.; Hicks, J.; Fox, M.; Thiessen, J. D.; Ronald, J. A.
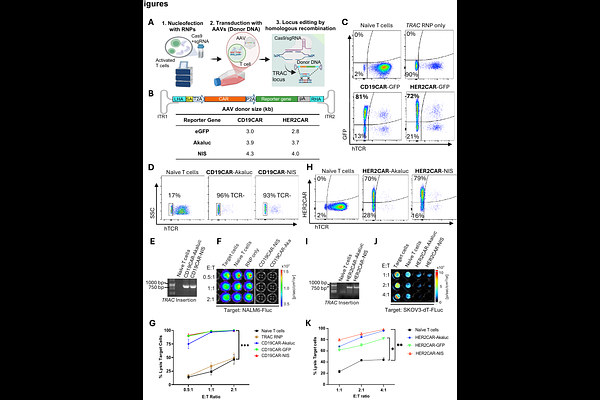
AbstractRationale: Chimeric antigen receptor (CAR) T cell therapies have shown remarkable success in treating hematological cancers and are increasingly demonstrating potential for solid tumors. CRISPR-based genome editing offers a promising approach to enhance the potency and safety of CAR-T cells. However, several challenges persist, including inefficient tumor homing and treatment-related toxicities in normal tissues, which continue to hinder widespread adoption. Advanced imaging technologies, including bioluminescence imaging (BLI) and positron emission tomography (PET), provide real-time insights into CAR-T cell distribution and activity in vivo, both in preclinical models and in patients. Here, we developed Trackable Reporter Adaptable CRISPR-Edited CAR (tRACE-CAR) T cells, a modular system for site-specific integration of CARs and imaging reporters. Methods: The luciferase reporter AkaLuciferase (AkaLuc) or the human sodium iodide symporter (NIS) were cloned downstream of the CAR in adeno-associated virus (AAV) donors for BLI or PET tracking, respectively. CARs with imaging reporters were knocked into the TRAC locus of primary human T cells via CRISPR editing and AAV transduction. Editing efficiency was evaluated by flow cytometry and junction PCR. In vitro cytotoxicity was assessed by BLI using firefly luciferase (Fluc)-expressing cancer cells co-cultured with CAR-T cells at varying effector-to-target ratios. In vivo, BLI and PET imaging assessed CAR-AkaLuc and CAR-NIS T cell expansion and trafficking in Nod-SCID-gamma mice bearing xenograft tumors. Results: T cell receptor (TCR) knockout efficiency exceeded 85%, with CAR expression observed in 70-80% of cells, depending on the reporter used. Reporter-engineered CAR-T cells retained functionality in vitro and exhibited significant cytotoxicity against target cancer cells, outperforming naive T cells. In vivo, AkaLuc BLI and 18F-tetrafluoroborate PET enabled non-invasive tracking of viable CAR-T cells. Notably, the route of administration (intravenous, peritumoral, or intraperitoneal) significantly influenced the distribution of CAR-T cells and their therapeutic effectiveness. Conclusion: tRACE-CAR enabled precise optical and PET tracking of CAR-T cells in models of B cell leukemia and ovarian cancer, allowing dynamic, non-invasive monitoring of cell distribution in both tumors and off-target tissues. This imaging platform could lead to more personalized, effective CRISPR-edited CAR cell therapies.