AD-genes and Aging Increase Count and Size of Lipid Droplets, Accompanied by Accumulation of Neutral Lipids Across Compartments in Hippocampal Neurons.
AD-genes and Aging Increase Count and Size of Lipid Droplets, Accompanied by Accumulation of Neutral Lipids Across Compartments in Hippocampal Neurons.
Benitez-Mata, B.; McWhirt, J.; Santana, R. A.; Brewer, G. J.; Digman, M. A.
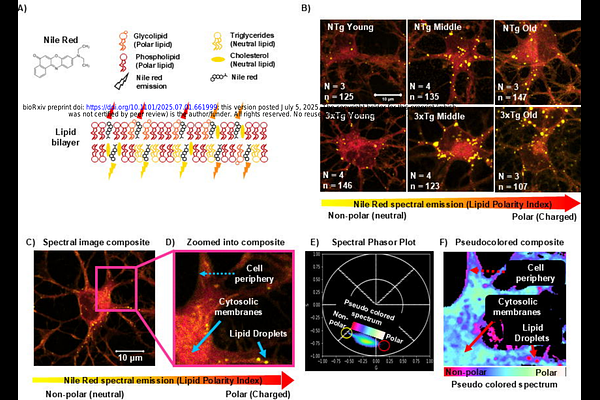
AbstractLipid homeostasis plays a crucial role in neuronal function, yet its dynamics during aging and neurodegenerative diseases remain poorly understood. Our study unveils critical age-related changes in lipid polarity and lipid droplet characteristics in hippocampal neurons from non-transgenic (NTg) mice and from an Alzheimer\'s disease-model (3xTg-AD). Using advanced spectral imaging and phasor analysis techniques, we tracked lipid polarity with Nile Red in vitro across various cellular compartments and quantified lipid droplet features. We discovered that NTg neurons exhibit a progressive increase in global lipid polarity from young to middle age, followed by a slight decrease in old age. This pattern suggests that neurons actively regulate their lipid composition throughout the lifespan, potentially in response to changing cellular needs. In contrast, AD-like (or 3xTg-AD) neurons fail to show this age-related increase in lipid polarity, instead displaying a consistent reduction in lipid polarity across all ages. Lipid droplet analysis revealed a transient accumulation of larger droplets in middle-aged NTg neurons, while AD-transgenic neurons showed early and persistent increases in lipid droplet size and number. Principal component analysis uncovered coordinated changes in lipid polarity and droplet characteristics, highlighting distinct patterns of lipid partitioning in NTg and AD-affected neurons. These findings suggest that AD-associated genetic modifications disrupt normal age-related adaptations in lipid metabolism and organization. Our results provide new insights into the complex interplay between lipid homeostasis, aging, and AD-genotypic stress. Understanding these dynamics may open new avenues for developing therapeutic strategies to maintain neuronal health and potentially slow AD progression.