Allosteric Modulation of MIF-2 Structure, Catalysis, and Biological Signaling via Cysteine Residues and a Small Molecule, Ebselen
Allosteric Modulation of MIF-2 Structure, Catalysis, and Biological Signaling via Cysteine Residues and a Small Molecule, Ebselen
Widjaja, V.; D'Orazio, S. M.; Das, P.; Takeda, X.; Rajendran, D. T.; Shi, Y.; Varghese, I.; Lam, Y.; Wang, J.; Batista, V. S.; Bhandari, V.; Lisi, G. P.
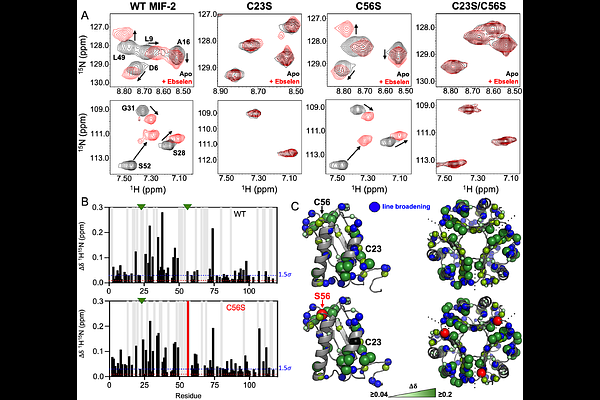
AbstractThe macrophage migration inhibitory factor (MIF) family of cytokines comprised of the MIF and D-dopachrome tautomerase (or MIF-2) paralogs share identical tertiary and quaternary structures that contribute to their overlapping enzymatic and signaling activities. Recent investigations of MIF and MIF-2 have shown them to possess N-to-C-terminal allosteric crosstalk, but despite the similarity of this \"allosteric pathway,\" its regulation of MIF and MIF-2 is not identical. Thus, structure alone does not preserve the precise allosteric mechanism and additional residues that modulate MIF and MIF-2 allosteric function must be characterized. Cysteines have been identified as allosteric switches for the same biochemical functions of MIF and small molecules targeting its N-terminal enzymatic site have affected the structure of three proximal cysteines. Ebselen is a compound that forms covalent selenylsulfide bonds with MIF cysteines and is hypothesized to destabilize and dissociate the MIF trimer into monomers. Ebselen-bound MIF also displays little-to-no catalysis or biological signaling. However, it is unclear whether Ebselen similarly affects the MIF-2 paralog, despite MIF-2 containing two related cysteines (MIF contains three). We used mutagenesis, nuclear magnetic resonance (NMR), molecular dynamics (MD) simulations, in vitro and in vivo biochemistry to investigate the mechanism of Ebselen as an allosteric modulator of MIF-2 via its cysteines. Our findings suggest that Ebselen partially disrupts the MIF-2 homotrimer, though the overall population of such a structure is <35%, even on the timescale of many hours. Ebselen does attenuate the biological functions of MIF-2 and solution structural biology captures the conformational transitions preceding the destabilized MIF-2 trimer.