The mPFC molecular clock mediates the effects of sleep deprivation on depression-like behavior and regulates sleep consolidation and homeostasis
The mPFC molecular clock mediates the effects of sleep deprivation on depression-like behavior and regulates sleep consolidation and homeostasis
Gardner, W.; Sarrazin, D. H.; Balzinger, M.; Marchese, C.; Ragno, A.; Ramanathan, C.; Veleanu, M.; Vestring, S.; Normann, C.; Bourgin, P.; Serchov, T.
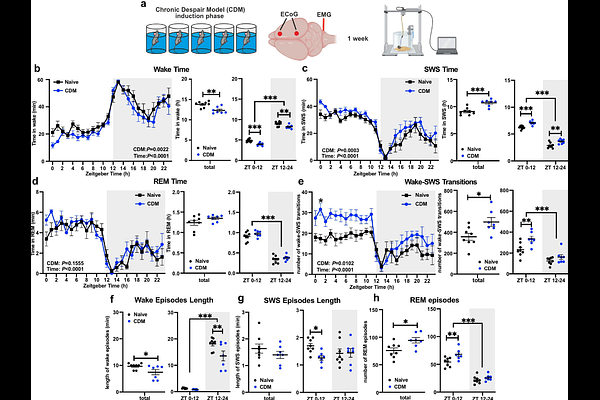
AbstractDisruptions in sleep, circadian rhythms, and neural plasticity are closely linked to the pathophysiology and treatment of depression. Acute sleep deprivation (SD) produces rapid but transient antidepressant effects, yet the underlying mechanisms remain poorly understood. Using a mouse model of stress-induced depression, we found altered sleep architecture, impaired sleep homeostasis, and disrupted day-night oscillations of the markers of glutamatergic plasticity - Homer1a and synaptic AMPAR expression in the medial prefrontal cortex (mPFC). These changes were accompanied by a blunted homeostatic response to SD. We further show that SD and ketamine, both rapid-acting antidepressants, exert opposing effects on mPFC circadian gene expression: SD enhances the expression of negative clock loop genes (e.g., Per, Cry), mirroring stress effects, while ketamine downregulates these same genes. Targeted deletion of the core clock gene Bmal1 in CaMK2a-expressing excitatory neurons of the mPFC disrupted sleep-wake architecture, elevated slow-wave activity, and abolished the behavioral and molecular (Homer1a) response to SD. Additionally, pharmacological activation of the clock repressor REV-ERB suppressed the antidepressant effects of SD. Our results demonstrate that the mPFC molecular clock is essential for the regulation of sleep consolidation and homeostasis, and mediates the effects of SD on behavior.