Selective targeting of IL-1RAP-dependent eosinophilic inflammation in allergic fungal airway disease
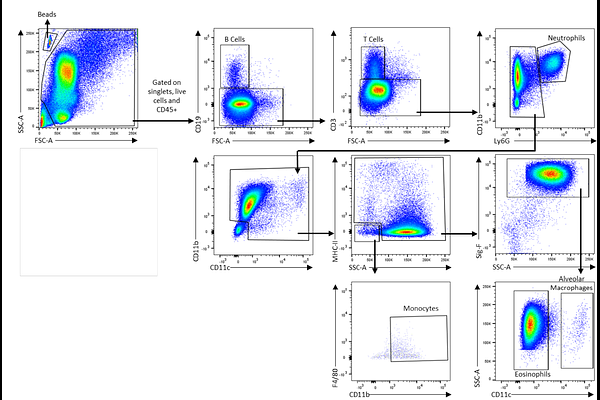
Selective targeting of IL-1RAP-dependent eosinophilic inflammation in allergic fungal airway disease
Williams, T. J.; Griffiths, J. S.; Gonzales-Huerta, L. E.; Bell, D.; Reed, A. K.; Shah, A.; Naglik, J. R.; Armstrong-James, D.
AbstractIt is estimated that in excess of 10 million people around the globe are affected by severe asthma and fungal sensitisation (SAFS) or allergic bronchopulmonary aspergillosis (ABPA), severe asthma endotypes driven by hypersensitivity to environmentally ubiquitous fungal pathogens, primarily Aspergillus fumigatus. Here, we sought to define the immunological pathways underlying these allergic fungal airway diseases. To do so, we exploit the chronic exposure repeat challenge model using live A. fumigatus conidia to systematically define the key immunological pathways driving airway inflammation in allergic fungal airway disease. In response to daily intranasal challenge, we observed increased absolute numbers of neutrophils and eosinophils in bronchoalveolar lavage fluid (BALF), characteristic of human allergic fungal airway disease, with significant depletion of the alveolar macrophage population. Transcriptomic analysis of BALF cells identified increased expression of IL-1 family cytokines and receptors including IL-1{beta}, IL-1RL1, IL-1R2 and the IL-18 binding protein. Complementary proteomic analysis of BALF revealed increased levels of cell death related proteins calprotectin and IL-1 Receptor Accessory Protein (IL-1RAP). Targeting IL-1RAP, using knockout mice, led to selective reduction in eosinophilia, IL-5 and IL-13 in the airways without impairment of fungal killing. This study identifies a role for IL-1RAP in the generation of eosinophilia independent of neutrophil influx and highlights its potential as a novel immunotherapeutic target for the treatment of allergic fungal airway disease.