Molecular determinants of extracellular TIMP-3 accumulation in Sorsby fundus dystrophy.
Molecular determinants of extracellular TIMP-3 accumulation in Sorsby fundus dystrophy.
Betts, J. H.; Hampton, K.; Strickland, D. K.; Clark, S. J.; Day, A. J.; Troeberg, L.
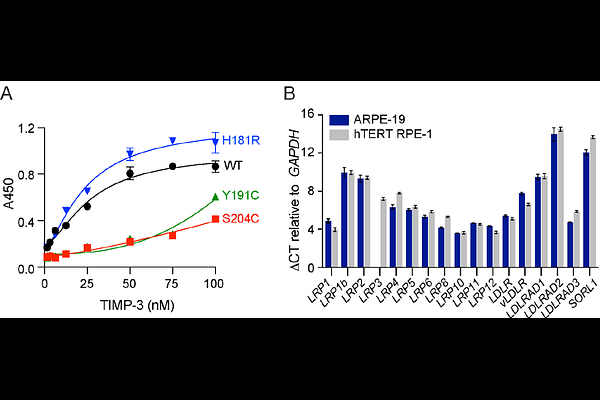
AbstractTissue inhibitor of metalloproteinases 3 (TIMP-3) is a critical regulator of extracellular matrix turnover. Mutations in TIMP-3 cause Sorsby fundus dystrophy, an inherited macular dystrophy that is similar to age-related macular degeneration (AMD), but which generally presents earlier. SFD is characterised by the accumulation of mutant TIMP-3 protein in Bruch\'s membrane, a multilamellar extracellular matrix underlying the retinal pigment epithelium (RPE). Here, we show that RPE cells regulate wild-type TIMP-3 levels post-translationally, with ARPE-19 and hTERT RPE-1 cells endocytosing the protein via the low-density lipoprotein receptor-related protein (LRP) family of scavenger receptors. LRP-mediated endocytosis of the SFD TIMP-3 variants S204C and Y191C was significantly delayed, establishing a molecular mechanism for their extracellular accumulation in SFD. In contrast, endocytosis of the SFD variant H181R TIMP-3 was unaltered, suggesting it accumulates through a distinct molecular mechanism, potentially via increased retention on extracellular matrix heparan sulfate proteoglycans. These findings reveal heterogeneity in the molecular mechanism of SFD pathogenesis, which has direct implications for therapeutic development. Genotype-specific interventions may be required, such as strategies to enhance receptor-mediated clearance or disrupt extracellular TIMP-3 retention. Our study also has broader implications for AMD, where TIMP-3 and other LRP and heparan sulfate ligands accumulate in drusen within Bruch\'s membrane. Age- and inflammation-dependent alterations in LRP expression and heparan sulfate structure may contribute to drusen formation and AMD progression. Understanding TIMP-3 trafficking in both physiological and pathological contexts could inform targeted treatments for SFD, AMD, and other degenerative disorders involving extracellular matrix dysregulation.