Mycobacterium tuberculosis effector protein PE5 hijacks the host CRL2 ubiquitin ligase complex
Mycobacterium tuberculosis effector protein PE5 hijacks the host CRL2 ubiquitin ligase complex
Madduri, B. T. S. A.; Vehra, O.; Han, A.; Radeny, J.; Resstel, C.; Bell, S. L.
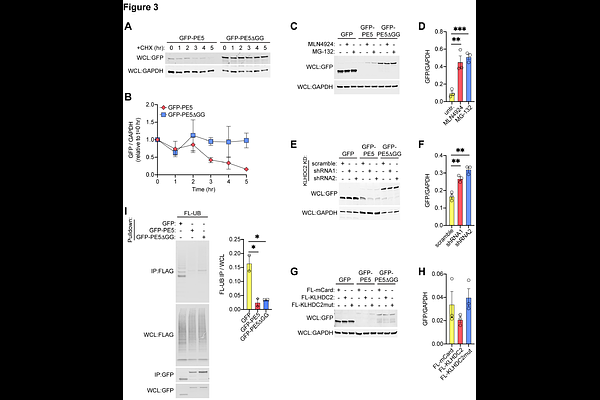
AbstractMycobacterium tuberculosis (Mtb) is the leading infectious killer and infects one quarter of the global population. During infection, Mtb evade host immune responses via secreted effector proteins that interfere with, modulate, and protect from potent antibacterial responses. One such class of mycobacterial effector proteins are the PE/PPE proteins, a huge family of proteins encoded by an impressive 10% of the Mtb genome. Because some PE/PPEs have demonstrated roles in immune regulation and host cell interaction, and because of the sheer number of PE/PPEs in pathogenic mycobacteria (~170-200+ members depending on the species), they are thought to contribute to Mtb virulence and pathogenesis. However, the cellular functions of Mtb\'s 169 PE/PPE proteins have yet to be comprehensively characterized, at least in part because of their high GC content and large regions of extremely repetitive regions found both in DNA and amino acid sequences. One member of this family, PE5, is likely critical for mycobacterial pathogenesis, but its cellular and molecular functions, particularly within a host cell, are unexplored. We investigated the molecular functions of PE5 using affinity purification coupled mass spectrometry (AP-MS) and identified an interaction with the CRL2 complex, a host E3 ubiquitin ligase complex. PE5 interacts with CRL2 through its C-terminal Gly-Gly motif, which is bound by the CRL2 substrate receptor, KLHDC2. PE5 does not get ubiquitinated, but it is degraded upon being bound by KLHDC2. Interestingly, binding to PE5 increases the autoubiquitination of KLHDC2, but this autoubiquitination does not interfere with KLHDC2\'s ability to degrade known substrates or the ability of CRL2 to degrade substrates bound by other substrate receptors. Therefore, while PE5 binds to CRL2-KLHDC2 and does not get ubiquitinated itself, it does not appear to impede CRL2 activity. This study identifies a novel interaction between PE5 and a host ubiquitin ligase pathway, and it raises new questions about how PE5\'s interaction with the host modulates cell biology to promote Mtb virulence. Furthermore, it implicates CRL2 complexes in mediating cell-intrinsic host response to Mtb infection, a novel function for this ubiquitin ligase complex. Our results expand our understanding of how PE/PPEs may target innate immune responses and contribute to our knowledge of how uncharacterized PE/PPEs contribute to Mtb\'s virulence.