Pcbp1 orchestrates amino acid metabolism burst during the naive-to-primed pluripotency transition
Pcbp1 orchestrates amino acid metabolism burst during the naive-to-primed pluripotency transition
Bakhmet, E. I.; Potapenko, E. V.; Shuvalov, O. Y.; Lobov, A. A.; Repkin, E. A.; Vorobyeva, N. E.; Korablev, A. N.; Zinovyeva, A. S.; Kuzmin, A. A.; Aksenov, N. D.; Kopylov, A. T.; Wu, G.; Schoeler, H. R.; Tomilin, A. N.
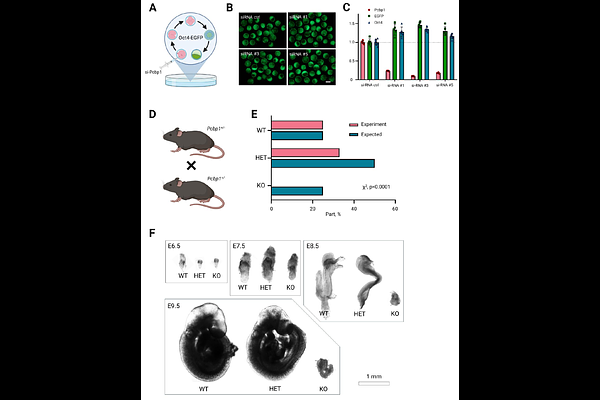
AbstractEmbryo implantation is accompanied by the naive-to-primed pluripotency transition in epiblast cells, making them receptive to external differentiation signals. In addition to this developmental program switch, implantation suggests that an anabolic boost is required for this process, as the embryo-uterine connection begins supplying the requisite nutrients. In this study, we show that the DNA-binding protein Pcbp1 plays a key role in intensifying amino acid metabolism during the priming of pluripotent stem cells. Knockout of the Pcbp1 gene leads to embryo growth arrest a few days after implantation. By modeling the naive-to-primed pluripotency transition in vitro, we observe reduced proliferation and induction of apoptosis in cells deficient for Pcbp1. Using multi-omics approaches, we uncover a crucial role for Pcbp1 in driving a transcriptional burst of numerous genes involved in the import and the de novo synthesis of essential and conditionally essential amino acids. Pcbp1 deficiency is consequently associated with a slowdown in protein biosynthesis, explaining the early lethal phenotype of knockout embryos. Our findings thus uncover the molecular mechanisms underlying anabolic changes during the naive-to-primed pluripotency transition and highlight the essential role of Pcbp1 in this process, also pointing to its functions in highly proliferative cells.