The structural basis of cold sensitivity
The structural basis of cold sensitivity
Choi, K. Y.; Lin, X.; Cheng, Y.; Julius, D.
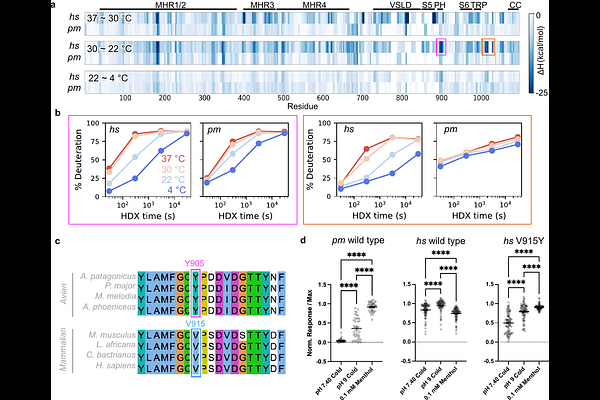
AbstractThermosensitive TRP ion channels enable somatosensory nerve fibers to detect changes in our thermal environment over a wide physiologic range. In mammals, the menthol receptor, TRPM8, is activated by temperatures below ~26{degrees}C and is essential for the perception of cold or chemical cooling agents. A fascinating, yet still unachieved goal is to elucidate structural mechanisms whereby TRPM8 or other thermosensitive channels are gated by changes in ambient temperature. Recent studies using cryogenic electron microscopy (cryo-EM) have attempted to address this challenging question but are limited by difficulties in visualizing temperature-evoked conformational substates or assessing the energetic landscape governing gating transitions. Here, we close this gap by using cryo-EM to visualize TRPM8 channels in cellular membranes, where bona fide menthol- and cold-evoked open states are captured. We identify a novel semi-swapped architecture in which interdigitation of channel subunits is substantially rearranged following repositioning of the S6 transmembrane helix and elements of the pore region. By combining this structural analysis with thermodynamic measurements using hydrogen-deuterium exchange mass spectrometry (HDX-MS), we are able to pinpoint the pore and TRP helices as key regions undergoing stimulus-evoked conformational dynamics that drive channel gating. Structural mechanisms associated with activation are validated by comparison of human TRPM8 with the menthol-sensitive, but relatively cold-insensitive avian orthologue. We propose a free energy landscape to explain channel gating by cold or cooling agents.