Constitutive activation and allosteric mechanisms underlying Gi/Gq signaling bias in SUCNR1 revealed by AlphaFold-based modeling and enhanced sampling simulations
Constitutive activation and allosteric mechanisms underlying Gi/Gq signaling bias in SUCNR1 revealed by AlphaFold-based modeling and enhanced sampling simulations
Shenol, A.; Petersen, J. E.; Lückmann, M.; Frimurer, T.; McCammon, J. A.; Schwartz, T. W.; Ma, W.
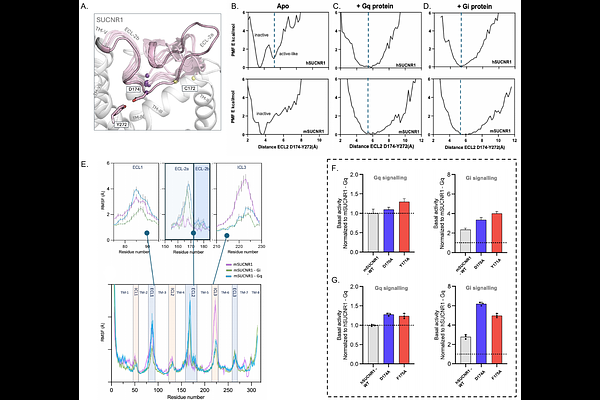
AbstractSuccinate Receptor 1 (SUCNR1) is a key metabolite sensing GPCR activated by high local succinate concentrations through Gi and Gq signaling pathways. By combining AlphaFold-based modeling, Gaussian accelerated molecular dynamics simulations (72s in total), receptor mutagenesis, and signaling assays, we here investigate the spontaneous and G-protein binding-associated activation mechanism of SUCNR1. Our molecular simulations predict that, in the absence of G protein or ligand, human SUCNR1 (hSUCNR1), in contrast to murine SUCNR1 (mSUCNR1), adopts canonical active-like conformations in its intracellular domains and undergoes critical conformational changes in its extracellular domain. This finding is consistent with our signaling-assay results showing that hSUCNR1, unlike mSUCNR1, displays a high degree of constitutive signaling. When complexed with either Gi or Gq, both h- and mSUCNR1 adopt highly energetically favorable conformations in the extracellular domain, characterized by the unlocking of extracellular loop 2b (ECL2b) and an expanded ligand entry pathway. Interestingly, the simulations reveal that helix 5 of Gq binds less firmly to the TM3/6 cleft of mSUCNR1, resulting in an unstable extracellular domain of the Gq-mSUCNR1 complex, which however can be stabilized by agonist binding. This result is supported by our BRET assays, which show that, in contrast to hSUCNR1, mSUCNR1 fails to recruit mini-Gq to the cell surface in the absence of an agonist. Furthermore, the signaling effects of key receptor residues at G-protein binding site and within ECL2b, as predicted by our simulations, were confirmed by mutagenesis assays. Overall, our integrative approach demonstrates that hSUCNR1 can spontaneously adopt all key conformations associated with receptor activation, and that G protein binding further primes the extracellular receptor domain for agonist binding, which in turn stabilizes the active complex.