Loss of meiotic double strand breaks triggers recruitment of recombination-independent pro-crossover factors in C. elegans spermatogenesis
Loss of meiotic double strand breaks triggers recruitment of recombination-independent pro-crossover factors in C. elegans spermatogenesis
Engebrecht, J.; Calidas, A.; Li, Q.; Ruiz, A.; Padture, P.; Barroso, C.; Martinez-Perez, E.; Silva, N.
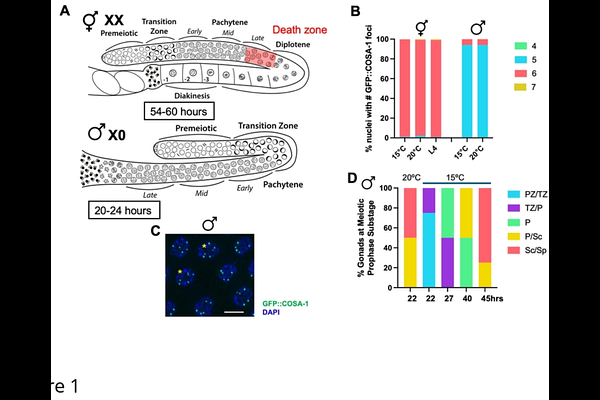
AbstractA key event in meiosis is the conversion of a small subset of double strand breaks into interhomolog crossovers. In this study, we demonstrate that Caenorhabditis elegans male spermatogenesis has less robust mechanisms than hermaphrodite oogenesis for ensuring and limiting the conversion of double strand breaks into crossovers. This is not a consequence of differences in meiotic prophase timing, sex chromosome genotype, or the presence or absence of germline apoptosis. Using the cyclin-like crossover marker COSA-1, we show that males have a linear response in converting increasing numbers of double strand breaks into crossovers, suggesting weakened crossover homeostasis. While the topoisomerase SPO-11, responsible for initiating meiotic double strand breaks, has an extended period of activity in males as in hermaphrodites, we discovered that COSA-1 foci form at the very end of meiotic prophase in the absence of SPO-11 during spermatogenesis. These COSA-1-marked sites are also independent of homologous recombination, and Topoisomerases I and II. We find that the synaptonemal complex, which holds homologs in proximity, differently modulates COSA-1 enrichment to chromosomes in the absence of SPO-11 in males and hermaphrodites. Together, these findings suggest that males have less robust crossover control and that there are previously unrecognized lesions or structures at the end of meiotic prophase in spermatocytes that can accumulate CO markers.