Structural Acrobatics of IAA7: Exercising Fuzzy Regions to Aid Auxin-Mediated Catalysis
Structural Acrobatics of IAA7: Exercising Fuzzy Regions to Aid Auxin-Mediated Catalysis
Parra, J. O. F.; Ott, M.; Bathia, T.; Emenecker, R.; Wolff, M.; Thalhammer, A.; Matschi, S.; Villalobos, L. I. A. C.
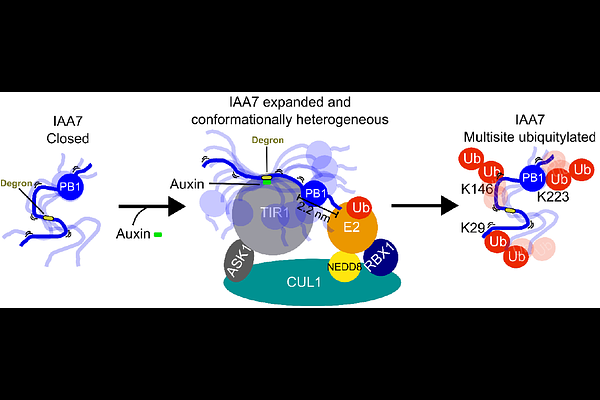
AbstractAuxin is the paragon of small molecules driving recognition of proteins for their ubiquitylation and degradation, enabling rapid decoding of external signals into transcriptional responses. Auxin functions as a molecular glue, enhancing interactions between the TRANSPORT INHIBITOR RESPONSE 1 (TIR1) and other TIR1-like proteins with various AUXIN INDUCIBLE/INDOLE-3-ACETIC ACID (AUX/IAA) transcriptional repressors. A Cullin RING E3 ubiquitin ligase, SCFTIR1/AFBs, mediates the ubiquitylation and degradation of AUX/IAA proteins by positioning them for ubiquitin transfer, leading to their rapid degradation by the 26S proteasome. AUX/IAAs contain a Phox Bem 1 (PB1) domain, a degron, and intrinsically disordered regions (IDRs) that influence auxin sensing and their polyubiquitylation by interacting with TIR1. However, the precise role of IDRs in AUX/IAA positioning for ubiquitylation, and the mechanism of multisite ubiquitylation within AUX/IAAs remain unclear. We used biophysical techniques and coarse-grained simulations (CGS) to study the structural behavior of IDRs in IAA7, a model AUX/IAA protein from Arabidopsis thaliana. Our findings show that IAA7 expands by approximately 2.6 nm upon binding to TIR1 in the presence of auxin, likely increasing the proximity between a lysine acceptor in IAA7 and the active site of the SCFTIR1 E3 ligase for ubiquitylation. Our data also suggest that IAA7 remains conformationally heterogeneous when bound to TIR1, forming numerous transient contacts along its IDRs and C-terminal PB1 with TIR1. We propose that these transient interactions outside the IAA7 degron interface enhance the TIR1-IAA7 association, resulting in highly dynamic and heterogeneous complexes that facilitate IAA7 multisite ubiquitylation, targeting it for rapid proteasomal degradation.