Dynamic balance of myoplasmic energetics and redox state in a fast-twitch oxidative glycolytic skeletal muscle fiber
Dynamic balance of myoplasmic energetics and redox state in a fast-twitch oxidative glycolytic skeletal muscle fiber
Disch, J.; Jeneson, J. A. L.; Beard, D. A.; Röhrle, O.; Klotz, T.
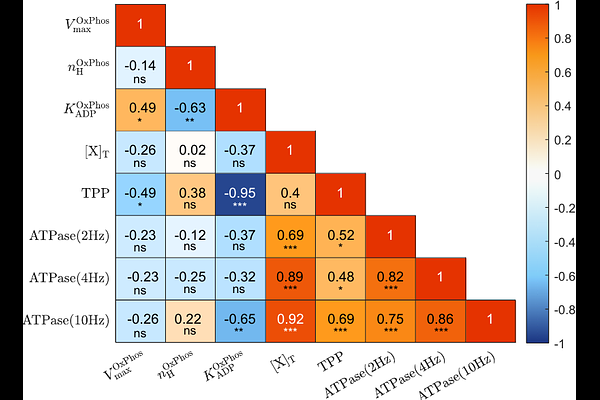
AbstractIn order to investigate the mechanisms governing energy and redox balance in skeletal muscle, we developed a computational model describing the coupled biochemical reaction network of glycolysis and mitochondrial oxidative phosphorylation (OxPhos) in fast-twitch oxidative glycolytic (FOG) muscle fibers. The model was identified against dynamic in vivo recordings of Phosphocreatine (PCr), inorganic Phosphate (Pi), and pH in rodent hindlimb muscle and verified against independent data from in vivo experiments and muscle biopsies. Step response testing revealed that mass action kinetics in combination with feedback control were sufficient to accomplish myoplasmic ATP homeostasis over a 100-fold range of ATP turnover rates. This vital emergent property of the metabolic model was associated with dynamic behaviour of intermediary metabolite concentrations similar to a second-order underdamped system that remains to be verified. The simulations additionally predicted that the lactate dehydrogenase (LDH) reaction makes substantial contributions to redox balance across the physiological range of ATP demands in this myofiber phenotype, while its role in slowing cellular acidification is minimal. Yet, LDH knock-out simulations revealed that oxidative recycling of myoplasmic NADH in and by itself sufficed to maintain redox balance over ATP turnover rates in the range of mitochondrial ATP synthesis. We conclude that aerobic lactate production in working muscles is a byproduct of the metabolic flexibility of FOG myofibers afforded by expression of high levels of LDH and OxPhos enzymes to support continual myoplasmic redox balance and ATP synthesis under conditions of high-intensity mechanical work. In the future, the presented simulation framework may be used to further enhance the understanding of how experimental observations in muscle emerge from the integrative behaviour of the metabolic network for carbohydrate metabolism in FOG myofibers.