Distinct substrate and intermediate recognition via mutation effects on Mycobacterium tuberculosis methionyl-tRNA synthetase
Distinct substrate and intermediate recognition via mutation effects on Mycobacterium tuberculosis methionyl-tRNA synthetase
THAKUR, S.; Mehra, R.
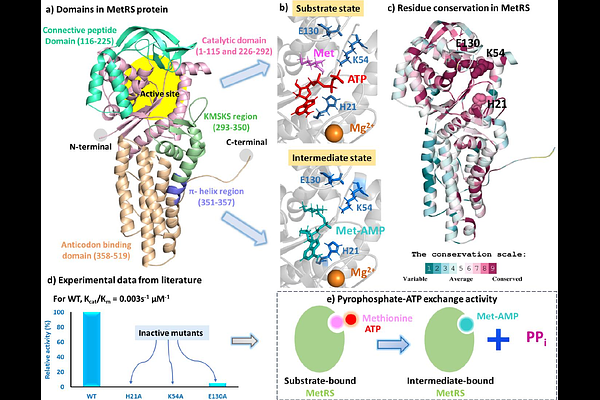
AbstractTuberculosis kills millions worldwide. Drug-resistance demands exploring new targets against this illness. Methionyl-tRNA synthetase (MetRS) is a crucial target in Mycobacterium tuberculosis (Mtb) that participates in initiation and elongation of translation and represents a protein of evolutionary interest. To elucidate the structure-function relationships of MetRS, we performed detailed sequence analyses and molecular dynamics simulations of Mtb MetRS in the substrate-bound (methionine and ATP) and intermediate (methionyl-AMP) states, for both the wild-type and three single-mutant forms (H21A, K54A, and E130A). Eight systems (two wild-type and six mutants) were simulated for 24 microseconds. Differential dynamics and binding effects of the substrate versus intermediate states were identified, along with the molecular reasons for the loss of activity in mutants. The wild-type substrate state was more stable than the intermediate state. In contrast, the mutants were more unstable in the substrate state, but incorporated stability into the intermediate state protein. These findings suggest that methionyl-AMP, being a reaction intermediate, exhibits a short residence time at the MetRS active site, while the substrate state shows a longer residence time of methionine and ATP. The increased instability of mutants in the substrate state indicates disruption of the pyrophosphate-ATP exchange by altering substrate-protein interactions. Once the intermediate is formed, the mutations have minimal or no effect. These observations are consistent with experimental data. In brief, our study finds the molecular basis for the distinct substrate and intermediate recognition by Mtb MetRS and establishes a mechanism for loss of activity in the mutants.