Architecture of the bundle-shaped phycobilisome from Gloeobacter violaceus
Architecture of the bundle-shaped phycobilisome from Gloeobacter violaceus
Burtseva, A.; Slonimskiy, Y.; Baymukhametov, T.; Sinetova, M.; Maksimov, E. G.; Popov, V. O.; Boyko, K. M.; Sluchanko, N. N.
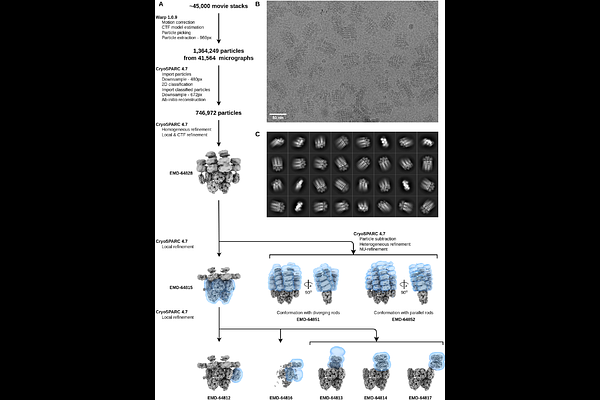
AbstractCyanobacteria optimize light harvesting for photosynthesis using large, soluble antenna systems called phycobilisomes (PBS). These megacomplexes, composed of spectrally distinct phycobiliproteins and linker proteins, allow efficient energy transfer to reaction centers of the photosystems. While PBS structures from many cyanobacteria have been resolved in recent years, the PBS of Gloeobacter violaceus - a phylogenetically ancient, thylakoid-less cyanobacterium - remains poorly characterized. Here, we present the cryo-EM structure of the G. violaceus PBS, a >10-MDa complex with a unique architecture consisting in 516 polypeptide chains totaling nearly 90,000 residues and harboring over 860 bilin chromophores. Unlike canonical PBS with radiating rods, the Gloeobacter PBS features diverging, conformationally flexible rod bundles formed by stacked phycoerythrin (PE) and phycocyanin (PC) hexamers. These rods emerge from a pentacylindrical allophycocyanin (APC) core, which is encircled by additional PC hexamers - a structural feature atypical for other cyanobacteria. We identify the role of the two Gloeobacter-specific multidomain linker proteins, Glr1262 and Glr2806, as critical stabilizers of this bundle-shaped architecture. Structural analysis suggests these linkers mediate rod-core interactions while permitting rod mobility, potentially balancing light capture and photoprotection. Furthermore, we demonstrate that the lateral cylinders of the APC core create alternative energy transfer pathways from the rods to the terminal emitters, ensuring robust excitation delivery under varying light conditions. This redundancy may represent an evolutionary adaptation to Gloeobacter\'s unique physiology. Our findings provide the high-resolution structural insight into Gloeobacter PBS, revealing divergent adaptations in early-branching cyanobacteria. The structure advances understanding of PBS diversity and evolution, offering a framework for bioengineering light-harvesting systems in synthetic biology and biotechnological applications.